
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-9-2017
Date: 19-1-2016
Date: 9-5-2016
|
ADDITION POLYMERIZATION
Addition polymerization is employed primarily with substituted or unsubstituted olefins and conjugated diolefins. Addition polymerization initiators are free radicals, anions, cations, and coordination compounds.
In addition polymerization, a chain grows simply by adding monomer molecules to a propagating chain. The first step is to add a free radical, a cationic or an anionic initiator (IZ) to the monomer. For example, in ethylene polymerization (with a special catalyst), the chain grows by attaching the ethylene units one after another until the polymer terminates. This type of addition produces a linear polymer:
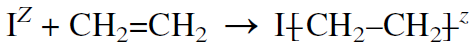
Branching occurs especially when free radical initiators are used due to chain transfer reactions (see following section, “Free Radical Polymerizations”). For a substituted olefin (such as vinyl chloride), the addition primarily produces the most stable intermediate (I). Intermediate (II) does not form to any appreciable extent:

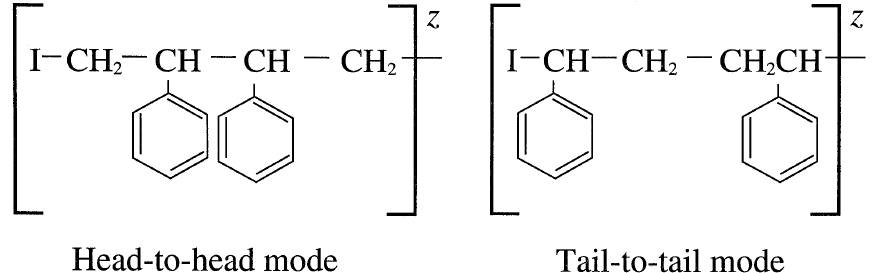
Propagation then occurs by successive monomer molecules additions to the intermediates. Three addition modes are possible: (a) Head to tail; (b) Head to head, and (c) tail to tail. The head-to-tail addition mode produces the most stable intermediate. For example, styrene polymerization mainly produces the head-totail intermediate:

Head-to-head or tail-to-tail modes of addition are less likely because the intermediates are generally unstable:
Chain growth continues until the propagating polymer chain terminates.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تستعد لإطلاق الحفل المركزي لتخرج طلبة الجامعات العراقية
|
|
|