


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-8-2017
Date: 13-2-2016
Date: 27-7-2017
|
Ethylbenzene
Ethylbenzene (EB) is a colorless aromatic liquid with a boiling point of 136.2°C, very close to that of p-xylene. This complicates separating it from the C8 aromatic equilibrium mixture obtained from catalytic reforming processes. Ethylbenzene obtained from this source, however, is small compared to the synthetic route.
The main process for producing EB is the catalyzed alkylation of benzene with ethylene:
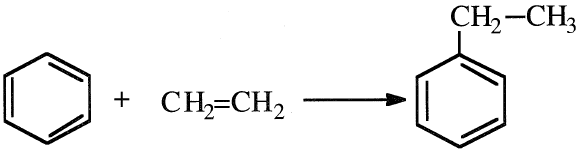
Many different catalysts are available for this reaction. AlCl3-HCl is commonly used. Ethyl chloride may be substituted for HCI in a molefor- mole basis. Typical reaction conditions for the liquid-phase AlCl3 catalyzed process are 40–100°C and 2–8 atmospheres. Diethylbenzene and higher alkylated benzenes also form. They are recycled and dealkylated to EB.
The vapor-phase Badger process (Figure 1.1), which has been commercialized since 1980, can accept dilute ethylene streams such as those produced from FCC off gas. A zeolite type heterogeneous catalyst is used in a fixed bed process. The reaction conditions are 420°C and 200–300 psi. Over 98% yield is obtained at 90% conversion.4,5 Polyethylbenzene (polyalkylated) and unreacted benzene are recycled and join the fresh feed to the reactor. The reactor effluent is fed to the benzene fractionation system to recover unreacted benzene. The bottoms containing ethylbenzene and heavier polyalkylates are fractionated in two columns.
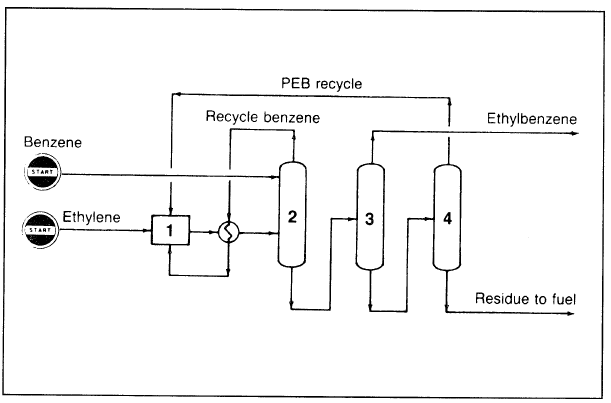
Figure 1.1. The Badger process for producing ethylbenzene: (1) reactor, (2) fractionator (for recovery of unreacted benzene), (3) EB fractionator, (4) polyethylbenzene recovery column.
The first column separates the ethylbenzene product, and the other separates polyethylbenzene for recycling. An optimization study of EB plants by constraint control was conducted by Hummel et al. They concluded that optimum operation could be maintained through a control system when conditions such as catalyst activity and heat transfer coefficients vary during operation.
Ethylbenzene is mainly used to produce styrene. Over 90% of the 12.7 billion pounds of EB produced in the U.S. during 1998 was dehydrogenated to styrene.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|