


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 25-7-2017
Date: 5-9-2017
Date: 14-9-2017
|
MECHANISM OF PROPENE OXIDATION
Much work has been invested to reveal the mechanism by which propylene is catalytically oxidized to acrolein over the heterogeneous catalyst surface. Isotope labeling experiments by Sachtler and DeBoer revealed the presence of an allylic intermediate in the oxidation of propylene to acrolein over bismuth molybdate. In these experiments, propylene was tagged once at Cl, another time at C2 and the third time at C3.
The formed acrolein was photochemically degraded to ethylene and carbon monoxide. It has been found that radioactivity was exclusively associated with ethylene when propylene tagged with 14C at C2 was used.
Also, carbon monoxide was found to be free from radioactivity:
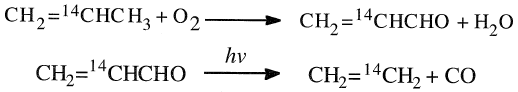
When propylene tagged with 14C at either Cl or C3 was oxidized to acrolein and then degraded, both CH2=CH2 and CO were radioactive, and the ratio of radioactivity was 1.
A proposed mechanism for the oxidation of propylene to acrolein is by a first step abstraction of an allylic hydrogen from an adsorbed propylene by an oxygen anion from the catalytic lattice to form an allylic intermediate:

The next step is the insertion of a lattice oxygen into the allylic species. This creates oxide-deficient sites on the catalyst surface accompanied by a reduction of the metal. The reduced catalyst is then reoxidized by adsorbing molecular oxygen, which migrates to fill the oxide-deficient sites. Thus, the catalyst serves as a redox system.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|