


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 4-5-2017
Date: 7-5-2017
Date: 25-2-2018
|
Molar Equilibrium Concentration
The molar equilibrium concentration, or just equilibrium concentration, refers to the molar concentration of a particular species in a solution at equilibrium. To specify the molar equilibrium concentration of a species, it is necessary to know how the solute behaves when it is dissolved in a solvent. For example, the molar equilibrium concentration of H2SO4 in a solution with a molar analytical concentration cH2SO4 = 1.0 M is actually 0.0 M because the sulfuric acid is completely dissociated into a mixture of H+, HSO4-, SO4-2 ions. There are essentially no H2SO4 molecules in this solution. The equilibrium concentrations of the ions are 1.01, 0.99, and 0.01 M, respectively.
Equilibrium molar concentrations are usually symbolized by placing square brackets around the chemical formula for the species. So, for our solution of H2SO4 with an analytical concentration of cH2SO4 51.0 M, we write

Example 1
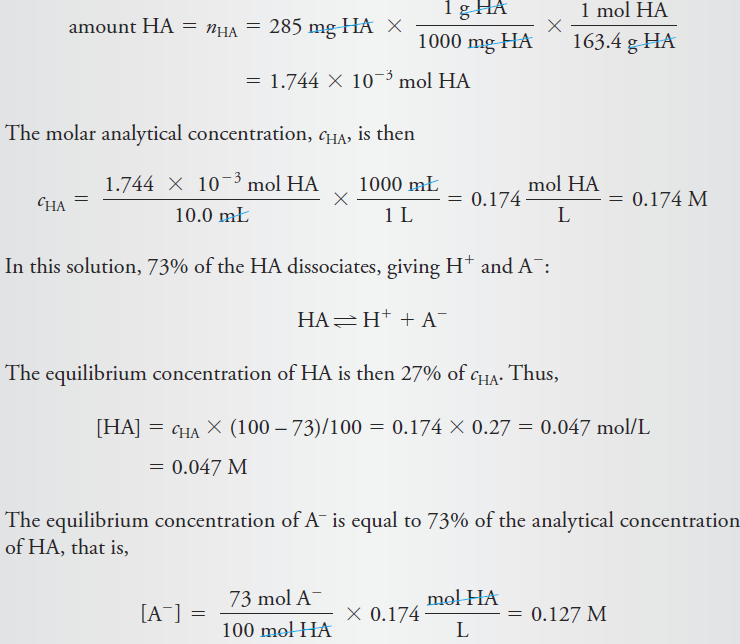
Calculate the analytical and equilibrium molar concentrations of the solute species in an aqueous solution that contains 285 mg of trichloroacetic acid, Cl3CCOOH (163.4 g/mol), in 10.0 mL (the acid is 73% ionized in water).
Solution

Example 2:

Example 3:
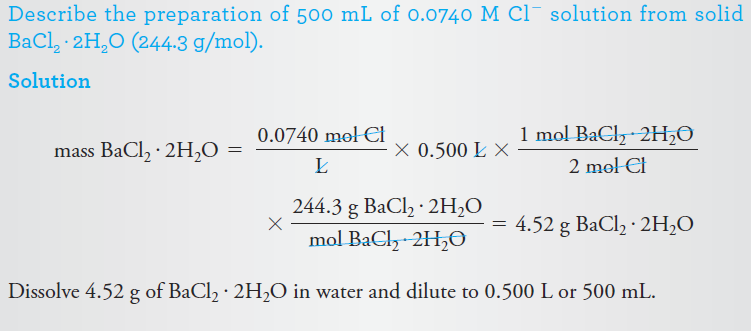



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
مكتب السيد السيستاني يعزي أهالي الأحساء بوفاة العلامة الشيخ جواد الدندن
|
|
|