

 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-2-2019
Date: 29-11-2018
Date: 29-11-2018
|
Occurrence The group 16 elements
Figure 1.1 illustrates the relative abundances of the group 16 elements in the Earth’s crust. Dioxygen makes up 21% of the Earth’s atmosphere, and 47% of the Earth’s crust is composed of O-containing compounds, e.g. water, limestone, silica, silicates, bauxite and haematite. It is a component of innumerable compounds and is essential to life, being converted to CO2 during respiration. Native sulfur occurs in deposits around volcanoes and hot springs, and sulfur-containing minerals include iron pyrites ( fool’s gold, FeS2), galena (PbS), sphalerite or zinc blende (ZnS), cinnabar (HgS), realgar (As4S4), orpiment (As2S3), stibnite (Sb2S3), molybdenite (MoS2) and chalcocite (Cu2S). Selenium and tellurium are relatively rare.

Fig. 1.1 Relative abundances of the group 16 elements (excluding Po) in the Earth’s crust. The data are plotted on a logarithmic scale. The units of abundance are parts per billion (1 billion =109). Polonium is omitted because its abundance is only 3 ×10-7 ppb, giving a negative number on the log scale.
Selenium occurs in only a few minerals, while Te is usually combined with other metals, e.g. in sylvanite (AgAuTe4).
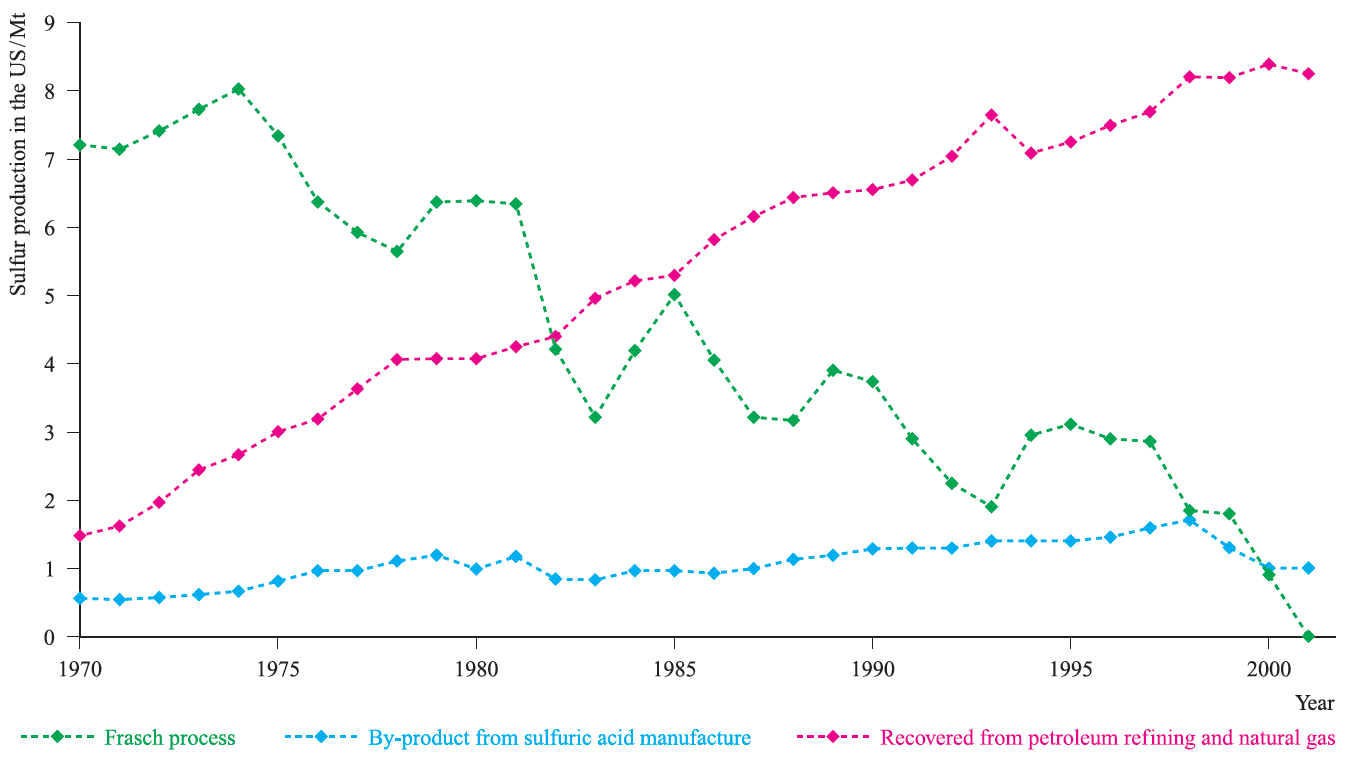
Fig. 1.2 Production of sulfur in the US from 1970 to 2001; note the increasing importance of recovery methods which have now replaced the Frasch process as a source of sulfur in the US. [Data: US Geological Survey.]



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|