


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-6-2019
Date: 23-2-2017
Date: 20-6-2019
|
Coordination number 8
As the number of vertices in a polyhedron increases, so does the number of possible structures (Figure 1.1a). Probably, the best known eight-vertex polyhedron is the cube, (1.1), but this is hardly ever observed as an arrangement of donor atoms in complexes. The few examples include the anions in the actinoid complexes Na3[PaF8], Na3[UF8] and [Et4N]4[U(NCS-N)8]. Steric hindrance between ligands can be reduced by converting a cubic into a square antiprismatic arrangement, i.e. on going from 1.1 to 1.2.
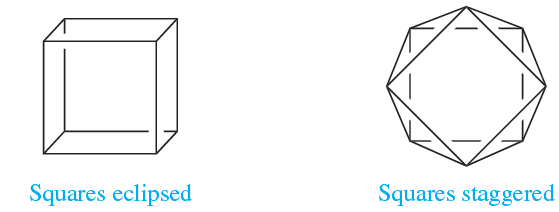
(1.1) (1.2)
Square antiprismatic coordination environments occur in [Zr(acac)4] (d0) and in the anions in the salts Na3[TaF8] (d0), K2[ReF8] (d1) and K2[H3NCH2CH2NH3][Nb)ox(4] (d1) (Figure 1.1b). Specifying the counter-ion is important since the energy difference between 8-coordinate structures tends to be small with the result that the preference between two structures may be altered by crystal packing forces in two different salts. Examples are seen in a range of salts of [Mo)CN(8]3- , [W(CN)8]3- , [Mo(CN)8]4- or [W(CN)8]4- which possess square antiprismatic or dodecahedral structures depending on the cation. Further examples of dodecahedral complexes include [Y(H2O)8]3+ (Figure 1.1c) and a number of complexes with didentate ligands: [Mo(O2(4]2- (d0), [Ti(NO3)4] (d0), [Cr(O2(4]3- (d1), [Mn(NO3)4]2- (d5) and [Fe(NO3)4]- (d5).
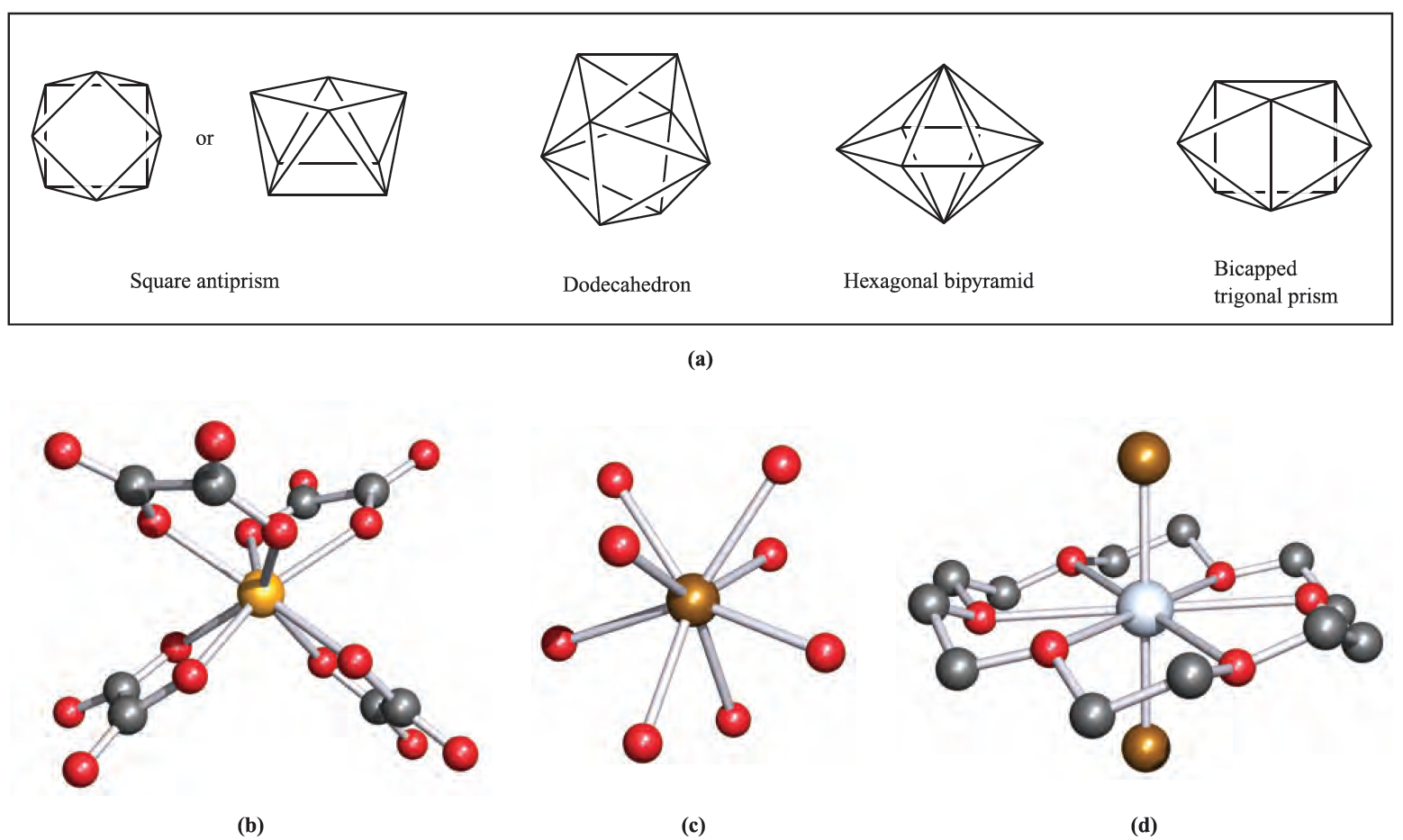
Fig. 1.1 (a) The coordination spheres defined by the donor atoms in idealized 8-coordinate structures; the left-hand drawing of the square antiprism emphasizes that the two square faces are mutually staggered. Examples of 8-coordinate complexes (X-ray diffraction): (b) the square antiprismatic structure of [Nb(ox)4]4- in the salt K2[H3NCH2CH2NH3][Nb(ox)4].4H2O [F.A. Cotton et al. (1987) Inorg. Chem., vol. 26, p. 2889]; (c) the dodecahedral ion [Y)H2O(8]3+ in the salt [Y(H2O)8]Cl3.(15-crown-5)[R.D. Rogers et al. (1986) Inorg. Chim. Acta, vol. 116, p. 171]; and (d) [CdBr2(18-crown-6(] with the macrocyclic ligand occupying the equatorial plane of a hexagonal bipyramid [A. Hazell (1988) Acta Crystallogr., Sect. C, vol. 44, p. 88]. Hydrogen atoms have been omitted for clarity; colour code: Nb, yellow; O, red; Y, brown; Cd, silver; C, grey; Br, brown.
The hexagonal bipyramid is a rare coordination environment, but may be favoured in complexes containing a hexadentate macrocyclic ligand, for example [CdBr2(18- crown-6)], Figure 1.1d. A bicapped trigonal prism is another option for 8-coordination, but is only rarely observed, e.g. in [ZrF8]4- (d0) and [La(acac)3(H2O)2].H2O (d0).



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|