


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 6-12-2020
Date: 30-12-2016
Date: 28-9-2020
|
Special Cases of 1st Law of Thermodynamics
1. Adiabatic Processes
Adiabatic processes are those that occur so rapidly that there is no transfer of heat between the system and its environment. Thus Q = 0 and
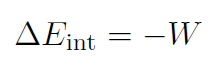
For example if we push in the piston very quickly then our work will increase the internal energy of the gas. It will store potential energy (ΔU = ΔEint) like a spring and make the piston bounce back when we let it go.
2. Constant-volume Processes
If we glue the piston so that it won't move then obviously the volume is constant, and  , because the piston can't move. Thus
, because the piston can't move. Thus
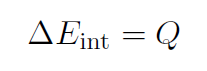
which means the only way to increase the internal energy of the gas is by adding heat Q.
3. Cyclical Processes
Recall the motion of a spring. It is a cyclical process in which the spring oscillates back and forth. After one complete cycle the potential energy U of the spring has not changed, thus ¢U = 0. Similarly we can push in the piston, then let it go and it will push back to where it started, similar to the spring. Thus ΔEint = 0 and
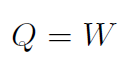
meaning that work done equals heat gained.
4. Free Expansion
Another way to get ΔEint = 0 is for
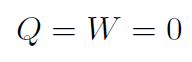



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|