
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Temperature Scales
المؤلف:
E. R. Huggins
المصدر:
Physics 2000
الجزء والصفحة:
473
6-12-2020
2687
Temperature Scales
We will put aside any worries about zero point energy, and simply assume that the temperature of an object is proportional to the average thermal kinetic energy of the molecules in the object, and that absolute zero is where no thermal kinetic energy remains.
From this point of view, the simplest way to define a temperature scale is to equate the temperature with the average thermal kinetic energy, and measure temperatur in energy units such as ergs as shown in Figure (1). But you probably have not heard anyone describe temperature in ergs, and for good reason. Telling your doctor that you are running a fever of 6.4 423 × 10–14, an increase of 23 × 10–18 over normal could be a bit hard to explain when you are sick. It is much easier to say that you have a temperature of 100° F or about 38°C. Ergs are too awkward a unit for most purposes. Historically, thermometers were invented and temperature scales established long before the relation between temperature and the average kinetic energy of molecules became known. Throughout the world the most widely used temperature scale is the Centigrade scale, where the temperature of melting ice is arbitrarily set at 0° C (zero degrees Centigrade), and the
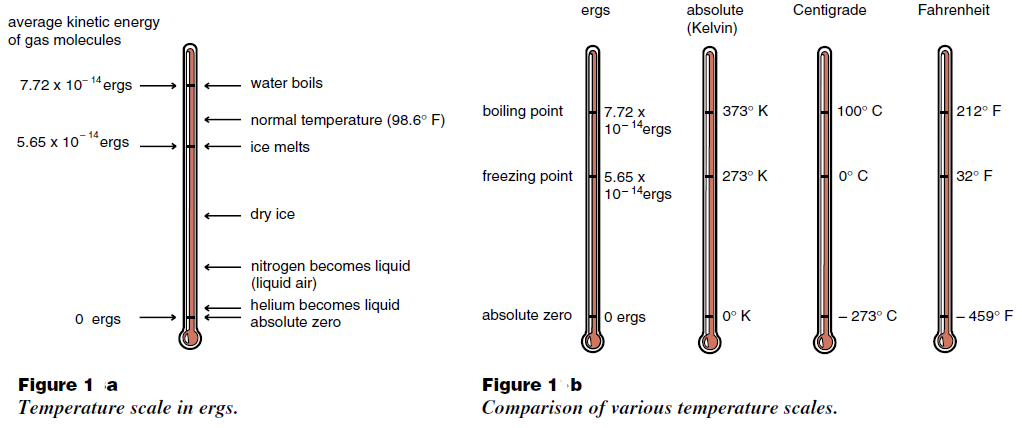
boiling of water at 100° C. Commonly, changes in temperature are measured with a mercury thermometer. This device registers temperature changes when the mercury in a thin glass column expands or contacts. On the Centigrade scale, the distance between 0° C and 100° C is marked into 100 equally spaced smaller intervals which we call degrees.
A less arbitrary scale is the Kelvin or absolute scale, which measures temperature in Centigrade size degrees beginning at absolute zero. Using the absolute scale, we find that helium boils at 4 degrees Kelvin, ice melts at 273 degrees Kelvin and water boils at 373 degrees Kelvin. A comparison of various temperature scales (ergs, degrees Kelvin, degrees Centigrade, and degrees Fahrenheit) is shown in Figure (1b).
Those who define standard nomenclature for physical quantities have decided, in their great wisdom, that the word “degrees” shall be omitted when talking about temperature in degrees Kelvin. Thus we should say that helium boils at 4 kelvins or 4K, ice melts at 273 kelvins or 273K, and the temperature difference between melting ice and boiling water is 100 kelvins or 100K. At least this nomenclature is easy to say and should not be confusing when you get used to it. We do not feel the same way about all recent changes in nomenclature.
The conversion from one temperature scale to another is a relatively straightforward process. If you went to an American school, somewhere along the way you were taught how to convert from Fahrenheit to Centigrade degrees. You do not need to worry about that because we will not be using the obsolete Fahrenheit scale. But we will often want to convert from the absolute scale to the energy units, ergs or joules. The conversion is written in the somewhat peculiar form
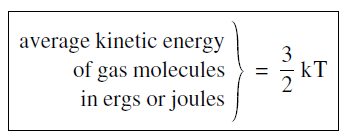 ........(1)
........(1)
where T is the temperature in kelvins, and the conversion factor k, known as Boltzman’s constant has the numerical value
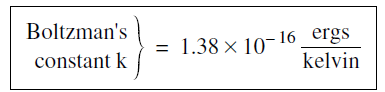 ...........(2)
...........(2)
If you are using MKS units, the value of k is 1.3 8 × 10–23 joules/kelvin.
The important feature of Equation 1 is that the average kinetic energy of the molecules is proportional to the absolute temperature measured in kelvins. We have written the proportionality constant as 3/2 k, putting in the numerical factor of 3/2 to get rid of a factor 2/3, as you will see shortly. Basically, think of Boltzman’s constant as the conversion factor to go from temperature
units to energy units or vice versa.
 الاكثر قراءة في الديناميكا الحرارية
الاكثر قراءة في الديناميكا الحرارية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















