


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 2-10-2016
Date: 12-11-2016
Date: 13-10-2016
|
Nuclear Decay
In the nucleus of an atom, neutrons and protons are held by nuclear forces. Their total energy (ignoring the mc2 contributions) is less than the barrier height potential energy. Yet some nuclear particles do escape. Any thoughts about the reason for an escape?
Answer
The wave function extends through the potential barrier to the outside world. Therefore the probability to be outside the nucleus is not zero. So why does the wave function itself extend into the barrier? All confinement problems, classical and quantum, have solutions with functions that extend into the barrier, usually decreasing exponentially to almost zero within a few wavelengths. Atomic particles have relatively long wavelengths compared to the barrier thickness. So why does the wave function itself not end in the barrier? Because the effective barrier height decreases with radial distance.
The probability to tunnel through the barrier is proportional to Exp 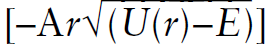 , where E is the energy of the incident particle, U(r) is the barrier potential as a function of distance r, and A is a constant that includes Planck’s constant h. Some closely related problems to be treated as tunneling through a barrier are:
, where E is the energy of the incident particle, U(r) is the barrier potential as a function of distance r, and A is a constant that includes Planck’s constant h. Some closely related problems to be treated as tunneling through a barrier are:
1. Bare copper wire is cut and the two ends are twisted together. In spite of the fact that the copper is coated with copper oxide, the twisted ends still conduct electricity readily.
2. Tunnel diode operation.
3. Scanning tunneling microscope.
For a discussion about how a nuclear decay rate may be influenced by its environment, see the Peres reference below.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|