
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Frenkel Defects
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المصدر:
A GUIDE TO PHYSICS PROBLEMS
الجزء والصفحة:
part 2 , p 45
29-8-2016
1614
Frenkel Defects
N atoms are arranged regularly to form a perfect crystal. If one replaces n atoms among them from lattice sites to interstices of the lattice, this becomes an imperfect crystal with n defects (of the Frenkel type). The number 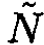 of interstitial sites into which an atom can enter is of the same order as N. Let ε0 be the energy necessary to remove an atom from a lattice site to an interstitial site. Show that, in the equilibrium state at temperature τ such that τ << ε0, the following relation is valid:
of interstitial sites into which an atom can enter is of the same order as N. Let ε0 be the energy necessary to remove an atom from a lattice site to an interstitial site. Show that, in the equilibrium state at temperature τ such that τ << ε0, the following relation is valid:
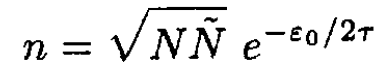
SOLUTION
We assume that the number of defects created around one lattice site does not affect the process of creating new defects. In other words, all configurations of the system are independent (not a very realistic assumption in general, but since τ << ε0 and the number of defects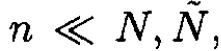 it can be used as an approximation). The vacancies and interstices then can be distributed in ωv and ωi ways, respectively:
it can be used as an approximation). The vacancies and interstices then can be distributed in ωv and ωi ways, respectively:
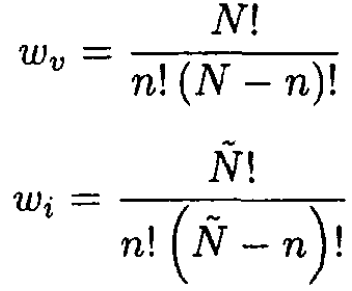 (1)
(1)
The total number of possible configurations of the system, ω, is given by
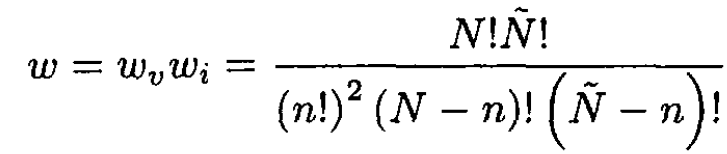 (2)
(2)
The entropy, S, may be written
 (3)
(3)
Using Stirling’s formula
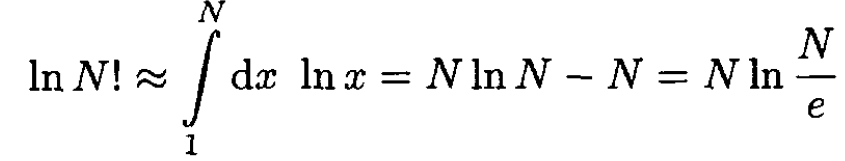 (4)
(4)
we obtain, from (3),
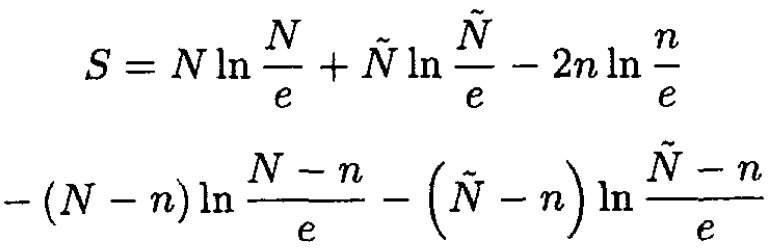 (5)
(5)
Using (5) and the fact that the total energy of the system E = nε0, we have
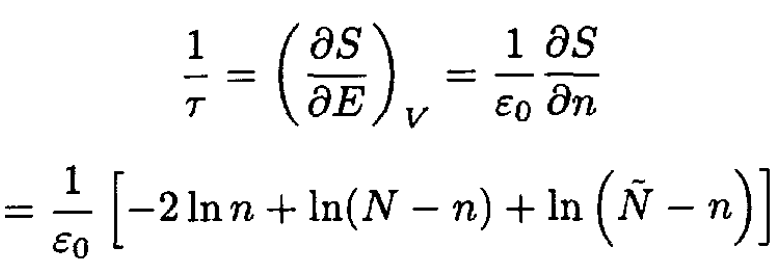 (6)
(6)
or
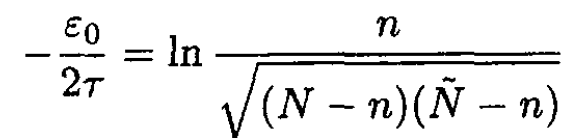 (7)
(7)
and
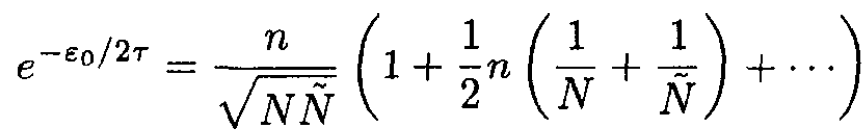 (8)
(8)
The condition ε << τ implies that 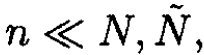 and therefore
and therefore
 (9)
(9)
 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















