
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Entropy of Mixing
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المصدر:
A GUIDE TO PHYSICS PROBLEMS
الجزء والصفحة:
part 2 , p 28
26-8-2016
1492
Entropy of Mixing
a) A 2-L container is divided in half: One half contains oxygen at 1 atm, the other nitrogen at the same pressure, and both gases may be considered ideal. The system is in an adiabatic enclosure at a temperature T = 293 K. The gases are allowed to mix. Does the temperature of the system change in this process? If so, by how much? Does the entropy change? If so, by how much?
b) How would the result differ if both sides contained oxygen?
c) Now consider one half of the enclosure filled with diatomic molecules of oxygen isotope 16O and the other half with 18O Will the answer be different from parts (a) and (b)?
SOLUTION
a) The energy of the mixture of ideal gases is the sum of energies of the two gases (since we assume no interaction between them). Therefore the temperature will not change upon mixing. The pressure also remains unchanged. The entropy of the mixture is simply the sum of the entropies of each gas (as if there is no other gas) in the total volume. We may write the total entropy S , as
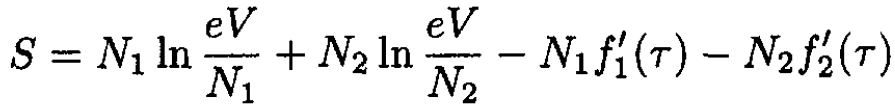 (1)
(1)
where N1 and N2 are the number of molecules of each gas in the mixture. V is the total volume of the mixture (V = V1 + V2). The entropy of the gases before they are allowed to mix is
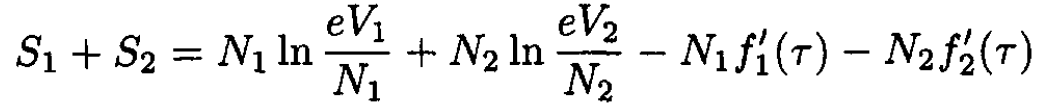 (2)
(2)
Therefore, the change in entropy, ∆S, is given by
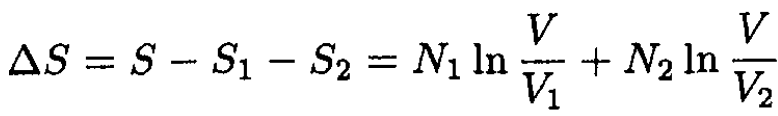 (3)
(3)
In our case V1 = V2 = V/2, and
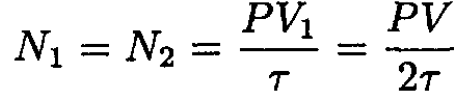
So, (3) becomes
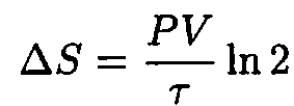 (4)
(4)
In conventional units we find
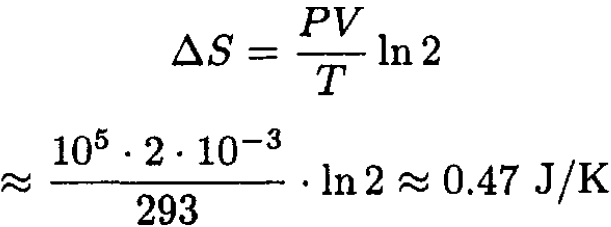 (5)
(5)
The entropy increased as it should because the process is clearly irreversible.
b) If the gases are the same, then the entropy after mixing is given by
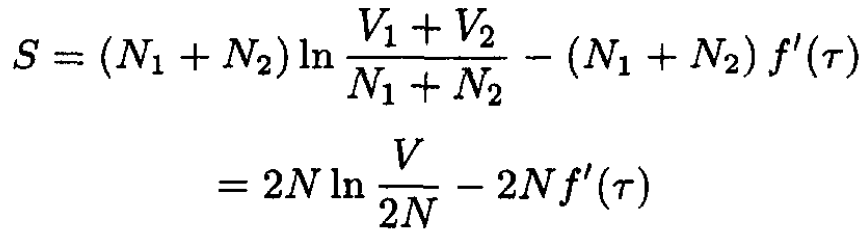 (6)
(6)
and so ∆S. In the case of identical gases, reversing the process only requires the reinsertion of the partition, whereas in the case where two dissimilar gases are mixed, some additional work has to be done to separate them again.
c) The same arguments as in (a) apply for a mixture of two isotopes,16O and 18O. The Gibbs free energy can be written in the form
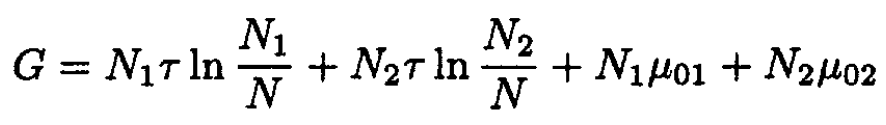 (7)
(7)
where μ01 and μ02 are the chemical potentials of pure isotopes. Therefore, the potential (7) has the same form as in the mixture of two different gases, and there is no correction to the result of (a). This is true as long as (7) can be written in this form, and it holds even after including quantum corrections to the order of h2.
 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















