
Ionizing Deuterium
 المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
 المصدر:
A GUIDE TO PHYSICS PROBLEMS
المصدر:
A GUIDE TO PHYSICS PROBLEMS
 الجزء والصفحة:
part 2 , p 78
الجزء والصفحة:
part 2 , p 78
 18-8-2016
18-8-2016
 1420
1420
Ionizing Deuterium
The hydrogen atom has an ionization energy of EH = 13.5983 eV when an electron is bound to a proton. Calculate the ionization energy of deuterium: an electron bound to a deuteron. Give your answer as the difference between the binding energy of deuterium and hydrogen (δE = ED – EH). The deuteron has unit charge. The three masses are, in atomic mass units, me = 5.4858 × 10-4, mp = 1.00728, md = 2.01355.
SOLUTION
The ionization energy of hydrogen is just the binding energy of the electron which is given in terms of the reduced mass μep of the electron–proton system. The same expression for deuterium contains the reduced mass μed of the electron–deuteron system:
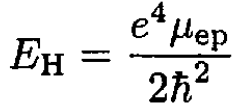 (1)
(1)
 (2)
(2)
 (3)
(3)
 (4)
(4)
The difference is easily evaluated. The ratio me/mp is a small number and can be used as an expansion parameter:
 (5)
(5)
The ratio of masses gives 2.72 × 10-4 and δE ≈ 3.700 meV.
 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


