


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 9-8-2016
Date: 13-7-2016
Date: 25-7-2016
|
Neutron Scattering
Neutrons can easily penetrate thick lead partitions but are absorbed much more efficiently in water or in other materials with high hydrogen content. Employing only classical mechanical arguments, give an explanation of this effect (see Figure 1.1).
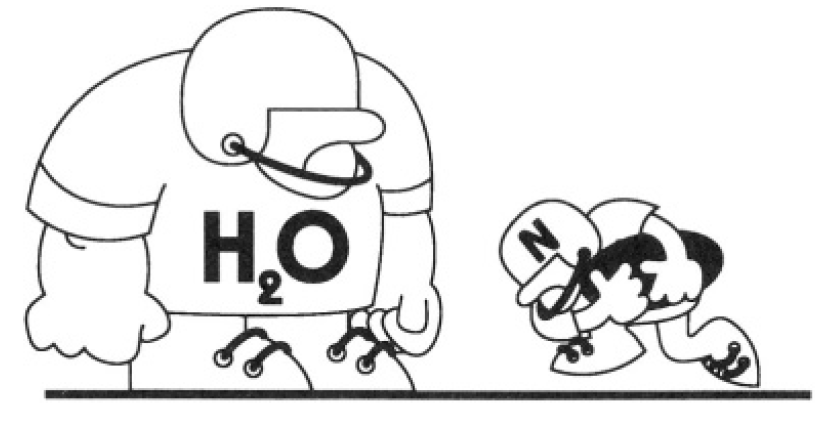
Figure 1.1
SOLUTION
Consider a neutron colliding with atoms of a certain type. In each collision, neutrons lose a fraction of their kinetic energy; let us calculate this fraction. We will assume that the collision is elastic and central. From energy and momentum conservation,
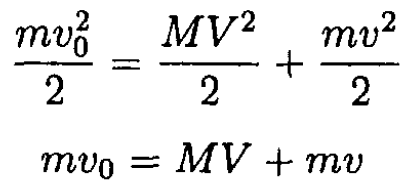
where m and M are the masses of the neutron and the atom, respectively; v0 and v are the initial and final velocities of the neutron; V is the velocity of the atom after the collision. These equations may be rewritten in the form
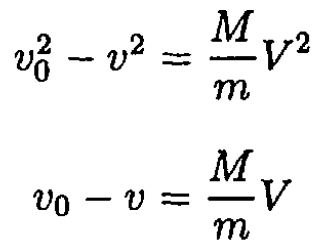
Solving for V gives
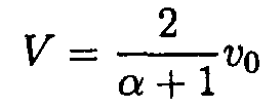
letting α = M/m. The kinetic energy of the atom after collision is
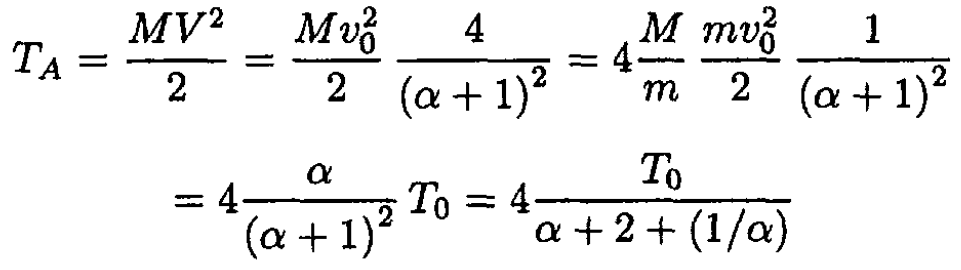
where T0 is the initial kinetic energy of the neutron. Obviously, as α → 0 or ∞, TA → 0. The maximum of TA as a function of α corresponds to the minimum of (α + 2 + 1/α) So we have α = 1(α positive). Here, m = M and the kinetic energy of the atom as a result of the collision will be a maximum TA = T0. For hydrogen, α = MH/m is very close to 1 and this explains why materials with high hydrogen content are so efficient in blocking the neutrons.



|
|
|
|
دراسة تكشف "مفاجأة" غير سارة تتعلق ببدائل السكر
|
|
|
|
|
|
|
أدوات لا تتركها أبدًا في سيارتك خلال الصيف!
|
|
|
|
|
|
|
مجمع العفاف النسوي: مهرجان تيجان العفاف يعزز القيم الأخلاقية والثقافية لدى طالبات الجامعات العراقية
|
|
|