


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-9-2019
Date: 21-10-2019
Date: 1-8-2018
|
Combined Inductive and Resonance Effects on Acid/Base Strength
When an acidic/basic functional group can interact with one or more substituents via a conjugated π system, a combination of inductive and resonance electron donating and withdrawing effects can occur. Resonance effects can arise from polarized substituents with π bonds such as C=O and S=O, which are resonance electron withdrawing, or from groups such as –NH2, –OR, and halides with lone pairs of electrons, which are resonance electron donating. Thus, the inductive and resonance effects of substituents like C=O and S=O act in concert to withdraw electron density from the conjugated X–H group. For substituents with lone pairs of electrons, however, inductive and resonance effects will oppose one another so that the overall effect will depend on which individual effect (inductive withdrawing or resonance donating) is stronger. When a substituent of either type is not fully conjugated with the acidic X–H group or not conjugated with it at all, the resonance contribution is substantially diminished and the overall effect will be dominated by the inductive effect of the substituent. We will now examine several examples to help illustrate these contrasting behaviors.
The effect of the nitro (NO2) group on the acidities of three different types of acidic functional groups is illustrated in Figure 1.1. The NO2 group is a π-containing substituent that is electron withdrawing via both inductive and resonance effects. Compared to the unsubstituted acids in the figure, the molecules that bear a nitro group have pKa values significantly lower. This indicates that the nitro-substituted analogs can better stabilize the deprotonated form of the acid (i.e., the conjugate base). Let us examine the source of this extra stabilization for the specific case of the para- and meta-substituted phenoxide anions.
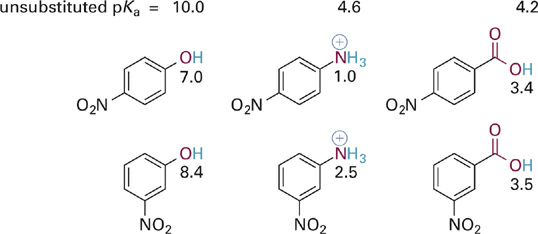
Figure 1.1 Illustration of the combined inductive and resonance effects of para- versus meta-nitro substitutions on the acidities of three different types of acidic groups—phenol, anilinium ion, and benzoic acids. The pKas of the unsubstituted parent compounds (without a nitro group) are given at the top for reference.
If we draw resonance hybrids of these two phenoxide anions, we observe that the para-substituted phenoxide possesses a particularly attractive resonance form (1.2). This is one in which a positive charge is positioned immediately adjacent to the anionic oxygen. We might draw an additional resonance form in which a C=O bond is formed between the atoms in question. What the resonance hybrid tells us is that electrons from the anionic oxygen atom can be fully delocalized throughout the ring and into the π system of the para-NO2 group as well, thus providing significant stabilization of the phenoxide anion. In contrast, the resonance hybrid for the meta-substituted analog does not allow for full delocalization of the negative charge on oxygen—one can “push electrons” from oxygen into the ring or from the ring onto the nitro group, but not both at the same time. The important thing to take away from this analysis is that the electron withdrawing effect of a meta-NO2 group (and of meta-substituted π-type substituents in general) involves mainly inductive and not resonance effects. In contrast, substitution at the para (or ortho) position with such groups involves a stronger electron withdrawing effect resulting from a combination of inductive and resonance effects.
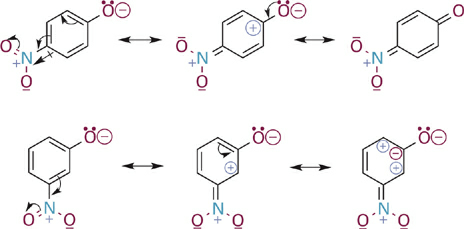
Figure 1.2 Comparison of key resonance structures for the para- and meta-nitro substituted phenoxide anions.
Next, let us consider the case of para- or meta-substitution with a methoxy group (OCH3) in an analogous series of acids (Figure 1.3). The methoxy group with its lone pair electrons is considered to exert a fairly strong resonance electron donating effect but also a fairly strong inductive electron withdrawing effect on account of the electronegativity of oxygen. In the examples at hand, the effect of para-methoxy substitution is electron donating (higher pKa values compared to the unsubstituted systems), indicating that resonance electron donation overwhelms the inductive withdrawing effect for para-substitution. In contrast, the overall effects of methoxy in the meta position is electron withdrawing (lower pKa values compared to unsubstituted systems), indicating that resonance electron donation is either not effective at this position or is much smaller than the inductive electron withdrawing effect.
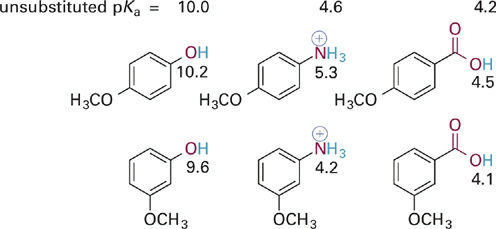
Figure 1.3 Comparison of the differences in the combined inductive and resonance effects of para- versus meta-methoxy substitutions on the acidities of three different types of acidic groups. The pKas of the unsubstituted parent compounds are given at the top for reference.
The above noted effect of the para-methoxy group on pKa can be understood by examining key resonance structures for the corresponding conjugate bases (Figure 1.4). Resonance donation of a lone pair of electrons into the aromatic π system places a negative charge in proximity to other negatively charged or electron-rich atoms. The net contribution of these resonance forms then is to destabilize the conjugate bases slightly, leading to the observed increases in pKa for the corresponding acid forms. The effect of meta-methoxy substitution is primarily inductive withdrawal of electrons, and is distance dependent—thus larger for compounds with fewer σ bonds between the substituent and the acidic X–H bond (Figure 1.5).
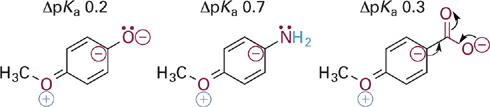
Figure 1.4 Comparison of key resonance structures for conjugate bases of three different para-OCH3 acids. The ΔpKa values refer to change in pKa compared to unsubstituted parent compound (ΔpKa = pKa-subst – pKa-parent)
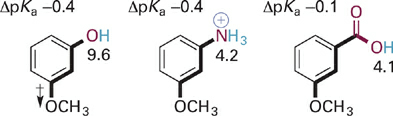
Figure 1.5 Meta-substituents primarily increase acidity through inductive electron withdrawal through σ bonds (bold bonds) and exhibit typical distance dependence.
The effects of other substituents bearing lone-pairs on electronegative atoms are demonstrated below in the context of phenolic and benzoic acids (Figure 1.6). The halogen atoms chlorine and fluorine are found to be slightly (for F) or moderately (for Cl) electron withdrawing at the para or ortho position, but most strongly withdrawing at the meta position. Why might this be so? The explanation is that these atoms exert a relatively modest resonance electron donating effect, less than the methoxy group but still significant. This resonance effect of the halogens is strongest at the ortho and para positions, for the same reason as it is in methoxy. Thus, the resonance effect partially offsets the dominant inductive withdrawing effect of the halogens when located at the ortho or para positions. At the metaposition, resonance effects are minimal (as is generally the case) and so the strong inductive effect dominates.
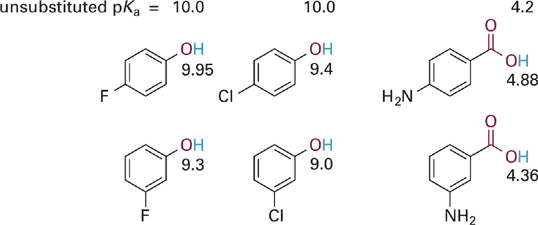
Figure 1.6 Comparison of the combined inductive and resonance effects of para- and meta-halides and amino groups on the acidities of acidic groups. The pKa values of the unsubstituted parent compounds are given at the top for reference and the pKa values of the substituted analogs are given near each structure.
Finally, comparison of the acidities for the meta- and para-aminobenzoic acids in Figure 1.6 shows that the intrinsically more basic N atom is overall electron donating in both positions, but as expected, is much more strongly donating at the para position. This is due to the full conjugation of the amino group with the π system of the COO− group, combined with a very weak inductive withdrawing effect. Amino groups are among the few substituents in which resonance electron donation dominates over inductive withdrawing effects, even at the meta position. Methyl groups are one of the few inductive electron donating groups, and since they have no resonance effect, a methyl (or other alkyl group) is electron donating at both the para and meta positions. This effect is similar to the amino group, albeit with a much smaller magnitude of electron donation.
To summarize, resonance electron withdrawal or donation is always strongest in the para and ortho positions because only in these positions can the substituent be fully conjugated with the acidic functional group. The four general categories of combined effects described in this section are as follows:
1. Substituents such as nitro with π bonds conjugated to aromatic rings, which are electron withdrawing by a combination of resonance and inductive effects. This effect is strongest at the para position.
2. Substituents such as the halides, which are strong inductive electron withdrawing groups at all positions, but are most withdrawing at the meta position. This is because of a weak and opposing electron donating resonance effect in the para or ortho position.
3. Other electronegative substituents such as OMe or OH are inductive electron withdrawing groups but have lone pairs of electrons that participate in resonance electron donation. These countering effects lead to an overall donating effect at the para and ortho positions, but an overall withdrawing effect at the metaposition.
4. Less electronegative substituents like amino groups (:NR2) have accordingly weaker inductive withdrawing properties and so act primarily as resonance electron donors. The effect is strongest at the para and ortho positions.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|