
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
MAGNETIC QUANTUM NUMBERS
المؤلف:
Mark Csele
المصدر:
FUNDAMENTALS OF LIGHT SOURCES AND LASERS
الجزء والصفحة:
p62
9-3-2016
2128
MAGNETIC QUANTUM NUMBERS
We have now described the orbital angular momentum of an electron with the term l, but an electron with angular momentum is analogous to a current loop and will exhibit a magnetic moment. This magnetic moment will (generally speaking) be characterized by the magnetic quantum number ml. An alternative way to think about this parameter is that it represents the direction of angular momentum of an electron. Assuming that we’ve chosen an axis for the atom, the orientation of this angular momentum (or orbital) with respect to this axis gives rise to ml. It can be thought of as the three-dimensional tilt of an elliptical orbit, as depicted in Figure1.1. This number is confined by the orbital quantum number l and may assume integer values ranging from -l to l. For example, an s orbital with l = 0 always assumes a magnetic quantum number of ml =0, while a p orbital with l =1 can have numbers ml = -1, 0, or 1. The range of numbers increases as the orbital does, with five values possible for a d orbital, and so on. In all, there are -l+1 possible values for ml for a given value of l.
It is somewhat puzzling perhaps why this is called the magnetic quantum number as opposed to tilt or some other description. The answer lies in the conditions

Figure 1.1. Representation of the magnetic quantum number.
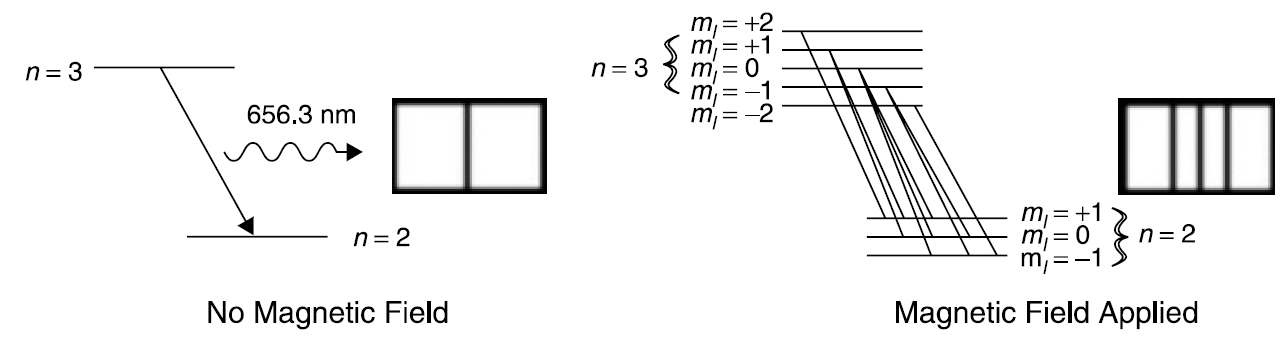
Figure 1.2. Zeeman effect on the red hydrogen line (energy levels and resulting spectra).
required to produce an energy difference between levels with various values for ml. Under the influence of a magnetic field, certain spectral emission lines can be split into a number of hyperfine lines in what is called the Zeeman effect. Figure 1.2 depicts the effect of an external magnetic field on the red emission line of hydrogen.
With no external magnetic field, the transition looks like a simple one between the n =3 and n =2 levels, resulting in the emission of a single wavelength. When a magnetic field is applied, the alignment of various magnetic moments becomes apparent as a number of spectra lines appear. Although it appears from Figure 1.2 that many hyperfine transitions are possible, there are selection rules that determine which transitions are unlikely (called forbidden) and which are allowed. The simple rule is that a transition is allowed (i.e., is quite probable) if the change in orbital angular momentum (l) is 21 or 1 (but not zero). A transition is also allowed if the change in the magnetic quantum number (ml) is -1, 0, or 1. Referring back to the figure, it is apparent that the transition from n =3, ml = +2 to n = 2, ml =+ 1 obeys these rules but that no other transition originating from the n = 3, ml =+2 level will. Furthermore, only three spectral lines are visible in the split because there are only three unique hyperfine transitions (i.e., changes in quantum numbers). Further application of selection rules can be seen in the spectrum of sodium observed, in which observed transitions correspond to a jump between an s and a p orbital (a change in l of 1) but never from a p to another p orbital.
 الاكثر قراءة في ميكانيكا الكم
الاكثر قراءة في ميكانيكا الكم
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















