


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-5-2017
Date: 23-9-2020
Date: 10-7-2018
|
Free-radical chain polymerization
This is perhaps the most well-known method of polymerization, and as the name implies, involves the continuous addition of monomer units to a growing freeradical chain. The general mechanism of this process in relation to the polymerization of a vinyl monomer is shown in Scheme 1. As Scheme 1 shows, initiation is a two-stage process in which, first a free radical is formed, and second this radical adds on to a monomer unit. The second stage is essentially the same for all the related processes; however, the first step can be achieved in a variety of ways; and the type of initiator depends on the nature of the polymerization experiment. In a laboratory, 2,2'-azo-bisisobutyronitrile (AIBN), in a sealed tube is usually the initiator of choice for this and other free-radical processes presumably because of the convenient timescale of its decomposition (Scheme 2) of about 18 h at 60 oC. More commonly used in an industrial setting are peroxides and, in an aqueous or mixed environment, inorganic initiators such as persulfate and other redox systems.
Electromagnetic radiation, usually visible or ultraviolet light but occasionally higher energy radiations such as X- and γ-rays are also of some importance, photoinitiators often being used to cure preformed polymer chains by the polymerization of pendant polymerizable side-groups. Some examples of free-radical initiators in common use are listed in Table (1) . In some cases, polymerization apparently occurs in the absence of any added initiator; here polymerization is induced by adventitious free-radical production.†
The propagation step is, of course, the core of the process, but as in all chaingrowth processes, it is the number of monomer units that are added for each initiator molecule that determines the molecular weight of the final material. In the case of free-radical polymerization this is controlled by considering the processes involved in terminating the growing chain; often these involve radical–radical combinations and high molecular weights are favoured by keeping the concentration of free radicals low. In the ideal case, that is, where there is no chain transfer, the number average degree of polymerization is related to the initiator concentration by eqn (1)
.
* It must also be noted here that not all photoinitiators initiate free-radical polymerization, for example, the use of onium salts as photoinitiators is based on their ability to initiate cationic polymerization processes.
† A bottle of styrene left untouched for long periods will be found to have polymerized even though the inhibitor has not been removed; monomer from which the inhibitor has been removed has an even shorter shelf-life. For this reason, it is suggested that styrene is disposed of after 12–18 months and that with the inhibitor removed used immediately.
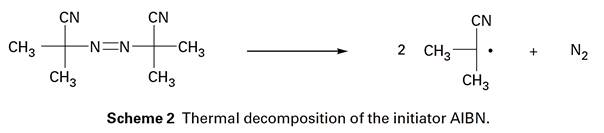
Table (1) Examples of free-radical initiators in common use

It is often found that the molecular weight of the material is rather higher than is convenient; in such cases chain-transfer agents may be used. Common chain-transfer agents in use include thiols and halogenoalkanes; and in some cases the solvent may be used to control molecular weight [e.g. both toluene and tetrahydrofuran (THF) may act in this way]. The use of functionalized chain-transfer agents such as 2-mercaptoethanol can lead to monofunctional polymers, as shown in Scheme 3; these can be subsequently reacted to form, for example, a block copolymer.




|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|