

 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 18-11-2015
Date: 19-11-2015
Date: 18-11-2015
|
Herpesviruses
The viruses in this family all feature a practically identical morphology, but show little uniformity when it comes to their biology and the clinical pictures resulting from infections. One thing shared by all herpesviruses is the ability to reactivate after a period of latency.
-The herpes simplex virus (HSV, two serotypes) is the pathogen that causes a vesicular exanthem (fever blisters, herpes labialis, or genitalis), encephalitis, and a generalized infection in newborns (herpes neonatorum).
-The varicella-zoster virus (VZV) causes the primary infection chickenpox, which can then recidivate as zoster (shingles).
-Cytomegalovirus (CMV) infections remain inapparent or harmless in the immunologically healthy, but can cause generalized, fatal infections in immunocompromised individuals.
-The Epstein-Barr virus (EBV) is the pathogen in infectious mononucleosis and is also implicated in lymphomas (including Burkitt lymphoma) and nasopharyngeal carcinomas.
-Human herpesvirus 6 (HHV 6) is the pathogen that causes three-dayfever (exanthema subitum, roseola infantum).
Human herpesvirus 8 (HHV 8) causes the AIDS-associated Kaposi sarcoma.
Diagnosis. Isolation, amplification culture, or direct detection can be used to diagnose herpes simplex, varicella-zoster, and cytomegaloviruses; antibody assays can be used for Epstein-Barr, human herpes 6 and 8, and varicella-zoster viruses; PCR can detect herpes simplex, varicella-zoster virus, cytomegalovirus, and human herpesvirus 6.
Therapy. Effective and well-tolerated chemotherapeutics are available to treat herpes simplex, varicella-zoster virus, and cytomegalovirus (acyclovir, ganciclovir).
Eight human herpesviruses that infect different organs are known to date, e.g., the skin (herpes simplex virus types 1 and 2, varicella-zoster virus), the lymphatic system (Epstein-Barr virus, human herpesvirus type 6, cytomegaloviruses), and the CNS (herpes simplex virus, cytomegalovirus).
Herpes simplex Virus (HSV)
Pathogen, pathogenesis, and clinical picture. The viral genome codes for about 90 proteins, categorized as “immediate early” (regulatory functions), “early” (DNA synthesis), and “late” (structural) proteins. Herpes simplex viruses are classified in types 1 and 2, which differ both serologically and biologically (host-cell spectrum, replication temperature). Initial infection with herpes simplex type 1 usually occurs in early childhood. The portal of entry is normally the oral mucosa (“oral type”) and the infection usually manifests as a gingivostomatitis. The viruses then wander along axons into the CNS, where they persist in a latent state in the trigeminal (Gasseri) ganglion. As with all herpesviruses, the pathogen remains in the macroorganism permanently after the primary infection. Following reactivation (endogenous recidivation), the viruses follow the same route back to the periphery, where they cause the familiar vesicular exanthem (“fever blisters,” herpes labialis, Fig. 1). Despite established immunity, such recidivations can manifest repeatedly because the viruses wander within the nerve cells and do not enter the intercellular space, thus remaining beyond the reach of the immune defenses. Possible complications include keratoconjunctivitis and a highly lethal form of encephalitis.
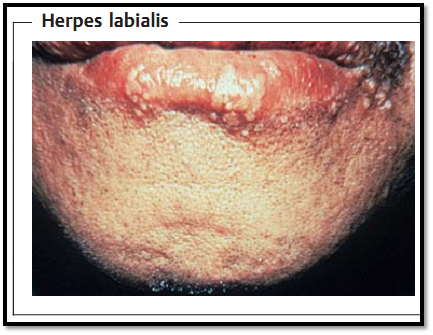
Fig.1 Following the initial infection, herpes simplex viruses (HSV) persist in the latent state in nerve cells of the CNS. When reactivated, they travel down the axons of these cells to the periphery, where they cause the typical vesicular exanthem.
The initial infection with HSV type 2 normally affects the urogenital area (“genital type”) and can be contracted despite an existing HSV type 1 infection. HSV type 2 persists in the latent state in the lumbosacral ganglia or peripheral tissues, from where it causes episodes of manifest herpes genitalis. Neurological complications are very rare and more benign than in HSV type 1. On the other hand, infections of newborn children (herpes neonatorum), e.g., in cases of maternal genital herpes, are feared for their high lethality rate.
Diagnosis. Cultivating the pathogen from pustule contents is the method of choice in labial and genital herpes. In an HSV encephalitis, the cerebrospinal fluid will contain few viruses or none at all. In such cases, they can only be cultivated from tissues (biopsy or autopsy material). Virus detection by means of cerebrospinal fluid PCR is worth a try.
Direct detection of the viruses under an electron microscope is only practicable if the specimen contains large numbers of viruses, which in practice will normally only be the case in blister contents. The virus can also be detected directly in patient specimens using immunofluorescence or in-situ hybridization (p. 408), but the material must contain virus-infected cells, i.e., blister contents are not as suitable here as in electron microscopy and virus isolation.
Serological investigation results in HSV lack significance due to the high level of general contamination in the population.
Epidemiology, prevention, and therapy. HSV type 1 is transmitted by 8 contact, and possibly by smear infection as well. Contamination with HSV therefore begins in early childhood. Transmission of HSV type 2 usually occurs during sexual intercourse, so that infections are generally not observed until after puberty. No immune prophylaxis (vaccination) is currently available for HSV. Acycloguanosine is used prophylactically in immunosuppressed patients.
Specific therapy is possible with acycloguanosine. Used in time, this chemotherapy can save lives in HSV encephalitis.
Varicella-zoster Virus (VZV)
Pathogen, pathogenesis, clinical picture. The VZ virus differs substantially from HSV, both serologically and in many biological traits. For instance, it can only be grown in primate cell cultures, in which it grows much more slowly and more cell-associated than is the case with HSV. No subtypes have been described.
The initial infection with VZV manifests in the great majority of persons as chickenpox, an episodic papulous exanthem. The portals of entry are the nasopharyngeal space and the conjunctiva. From there, the virus undergoes a viremic phase in which it is transported by the blood to the skin, where the typical exanthem is produced. The disease confers an effective immunity. In immunodeficient patients, a VZV infection (or reactivation, see below) can affect other organs (lungs, brain) and manifest a severe, frequently lethal, course.
After the symptoms of chickenpox have abated, the VZV persists in the spinal ganglia and perhaps in other tissues as well. Following reactivation, zoster (shingles) develops (Fig. 2), whereby the virus once again spreads neurogenically and causes neuralgia as well as the typical zoster efflorescence in the skin segment supplied by the sensitive nerves. Reactivation is induced by internal or external influences and becomes possible when cellular VZV immunity drops off, i.e., after about the age of 45 assuming normal immune defenses.
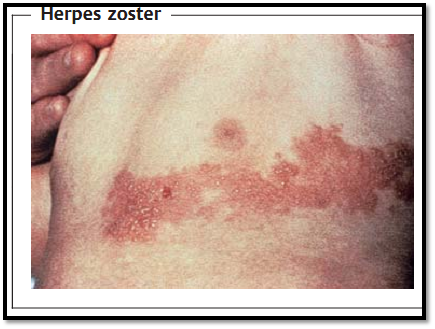
Fig. 2 The varicella zoster viruses (VZV) persist in the latent state in spinal ganglia cells. When reactivated, they cause dermal efflorescences in the corresponding dermatome.
Diagnosis. VZV can be detected with a wide spectrum of methods, namely PCR, isolation, direct viral detection by means of electron microscopy, detection of viral antigens using immunofluorescence in tissue specimens or cell smears, and serologically based on antibody titer increases or IgM detection.
Epidemiology, prevention, and therapy. VZV is highly contagious and is transmitted aerogenically. The primary infection, which manifests as chickenpox, is still almost exclusively a childhood disease today. A vaccine containing attenuated viruses is available for prevention of chickenpox and possibly zoster, but its use is currently a matter of controversy. In immunosuppressed patients, hyperimmunoglobulin can be used for passive immunization or postexposure immunity. Acycloguanosine is used both prophylactically and in treatment of VZV infections.
Cytomegalovirus (CMV)
Pathogen, pathogenesis, clinical picture. CMV is characterized by a narrow spectrum of hosts, slow replication, frequently involving formation of giant cells and late, slow development of cytopathology.
An initial infection with cytomegaly is inapparent in most persons, even in very early—perinatal or postnatal—infections. The virus apparently persists in the latent state in mononuclear cells. Reactivation can also run an asymptomatic course, but symptoms may also develop that are generally relatively mild, such a mononucleosislike clinical pictures, mild forms of hepatitis or other febrile illnesses. Droplet infection is the most frequent route of transmission, but smear infections and nursing infections are also possible. Generalized contamination with this pathogen (over 90% of the adult population is infected), frequent reactivation with, in some cases, months of continued excretion of viruses in saliva and urine and the wide variety of potential clinical pictures are all factors that often make it difficult to implicate CMV as the etiological cause of an observed illness. The virus infection can manifest as a sequel instead of a cause, for instance of a flulike illness. To labor the point somewhat, it could be said that the patient is not primarily ill due to a CMV infection, but rather has a florid CMV infection because he or she is ill.
The situation is different in AIDS, transplantation or malignancy patients, in whom a fresh CMV infection or reactivation—similarly to HSV and VZV— can result in severe generalized infections with lethal outcome. The liver and lungs are the main organs involved. Retinitis is also frequent in AIDS patients. In kidney transplant patients, a CMV infection of the mesangial cells can result in rejection of the transplant. Another feared CMV-caused condition is an intrauterine fetal infection, which almost always results from a primary infection in the mother: in 10% of cases the infection results in severe deformities.
Diagnosis. Amplification cultures from saliva, urine, buffy coat, tissue, or BAL (bronchoalveolar lavage) are a suitable method of confirming a florid CMV infection. In transplantation patients, the risk of a CMV manifestation can be estimated by immunocytochemical monitoring of the CMV- positive cell count in the peripheral blood (“antigenemia test”), since this count normally rises several days before clinical manifestations appear. Based on such an early warning, antiviral therapy can be started in time (ganciclovir, foscarnet). PCR results must be interpreted with a clear idea of how sensitive the method used can be, since the numbers of viruses found maybe clinically insignificant. Hasty conclusions can result in “overdiagnosis,” above all in CMV-positive transplant recipients.
Serological results are hardly useful in clarifying a florid infection due to the high level of generalized contamination. Added to this is the fact that the immunoincompetent patients in whom diagnosis of this infection would be particularly important are serologically problematical anyway. Serology does contribute to clearing up the CMV status of transplant recipients and donors.
Epidemiology, prevention, and therapy. CMV is transmitted by contact or smear infection, usually in childhood or adolescence. Immunosuppressed patients can be treated with hyperimmunoglobulin to provide passive immunity against infection or recidivation. Ganciclovir and foscarnet are therapeutically useful in transplantation, and particularly in AIDS patients, to combat CMV-induced pneumonia, encephalitis, and retinitis.
Epstein-Barr Virus (EBV)
Pathogen, pathogenesis, clinical picture. EBV infects only a narrow spectrum of hosts and replicates very slowly. It persists in a latent state in B lymphocytes and can lead to their immortalization and tumor transformation.
EBV enters the body through the mucosa. It replicates in epithelial cells of the oropharynx or cervix and enters B lymphocytes, where it continues to replicate. This results in the clinical picture of infectious mononucleosis (kissing disease or Pfeiffer disease), which is characterized by fever and a generalized but mainly cervical swelling of the lymph nodes, typically accompanied by tonsillitis, pharyngitis, and some cases of mild hepatic involvement. This virus also persists in latency, probably for the life of the patient, in (immortalized) B cells.
EBV and EBV-specific sequences and antigens are isolated in cases of Burkitt lymphoma and nasopharyngeal carcinoma. The higher incidence of Burkitt lymphoma in parts of Africa is attributed to a cofactor arising from the hyperendemic presence of malaria there. EBV exacerbates the B-cell proliferation resulting from a malaria infection. EBV has also been implicated in Hodgkin disease and T-cell lymphomas. These tumor forms also result from the interaction of EBV with other mechanisms of cell damage. In immunocompetent persons, the following lymphoproliferative diseases are sequelae of EBV infections:
-a benign polyclonal B-cell hyperplasia,
-its malignant transformation into a polyclonal B-cell lymphoma, and
-a malignant, oligoclonal or monoclonal B-cell lymphoma.
Diagnosis. Heterogenetic antibodies that agglutinate the erythrocytes of several animal species and antibodies to a variety of viral antigens are found in acute infectious mononucleosis:
-VCA (viral capsid antigen). Antibodies to VCA appear early and persist for life.
-EA (early antigen). Antibodies to EA are only detectable during the active disease.
-EBNA (Epstein-Barr nuclear antigen). Antibodies to EBNA are not produced until two to four weeks after disease manifestation, then persist for life.
Chronic mononucleosis is characterized by antibodies to VCA and EA.
The diagnostic procedures in lymphoproliferative diseases (see above) involve histology and cellular immunotyping.
Epidemiology, prevention, and therapy. EBV is excreted in saliva and pharyngeal secretions and is transmitted by close contact (“kissing disease”). As with all herpesviruses the level of generalized contamination is high, with the process beginning in childhood and continuing throughout adolescence. Neither immunoprophylactic nor chemoprophylactic measures have been developed as yet. Lymphoproliferative diseases involving viral replication can be treated with acyclovir and ganciclovir.
Human Herpesvirus (HHV) 6
Pathogen, pathogenesis, clinical picture. HHV-6 was isolated in 1986 in patients suffering from lymphoproliferative diseases and AIDS. The virus shows T-cell tropism and is biologically related to the cytomegalovirus.
HHV-6 exists in two variants, HHV-6A and HHV-6B. The pathogenic implications of their reactivation have not yet been described.
HHV-6B is the causal pathogen in exanthema subitum (roseola infantum), a disease that is nearly always harmless, characterized by sudden onset with high fever and manifests as a typical exanthem in small children. Reports of HHV-6-caused illness in adults are rare and the clinical pictures described resemble mononucleosis (EBV-negative mononucleosis). Apparently, how- 8 ever, this virus can also cause severe infections in bone marrow transplant patients (pulmonary and encephalitic infections). HHV-6A has not yet been convincingly implicated in any clinical disease.
Diagnosis and epidemiology. HHV-6 can be cultured in stimulated umbilical lymphocytes. Potentially useful diagnostic tools include antibody assay and PCR.
Generalized contamination with HHV 6 begins in early childhood, eventually reaching levels exceeding 90% in the adult population. The virus persists in latent form in the salivary gland, so that mother-to-child transmission is most likely to be in saliva.
Human Herpesvirus (HHV) 8
Pathogen, clinical picture. HHV 8 has recently been identified as a decisive cofactor in induction of Kaposi sarcoma. The classic, sporadic form of this malignancy was described in 1872 in the Mediterranean area. It also occurs
following organ transplantations and is a significant cause of death in AIDS patients (12%).
The contribution of HHV 8 to the pathogenesis of Kaposi sarcoma appears to lie in dysregulation of cytokine and hormone production. In transplantation-association Kaposi sarcoma the virus can also be transmitted by the transplant.
Diagnosis. Antibody assay (EIA, IF, Western blot).



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مستشفى العتبة العباسية الميداني في سوريا يقدّم خدماته لنحو 1500 نازح لبناني يوميًا
|
|
|