


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 27-8-2021
Date: 7-12-2021
Date: 26-9-2021
|
Eukaryotic Fatty Acid Synthase
The remaining series of reactions of fatty acid synthesis in eukaryotes is catalyzed by the multifunctional, homodimeric enzyme fatty acid synthase (FAS). The process involves the addition of two carbons from malonyl CoA to the carboxyl end of a series of acyl acceptors.
Each FAS monomer is a multicatalytic polypeptide with six different enzymic domains plus a 4ʹ- phosphopantetheine-containing acyl carrier protein (ACP) domain. 4ʹ- Phosphopantetheine, a derivative of pantothenic acid (vitamin B5), carries acyl units on its terminal thiol (–SH) group and presents them to the catalytic domains of FAS during fatty acid synthesis. It also is a component of CoA. [Note: In prokaryotes, FAS is a multienzyme complex.] The reaction numbers in brackets below refer to Figure 1.
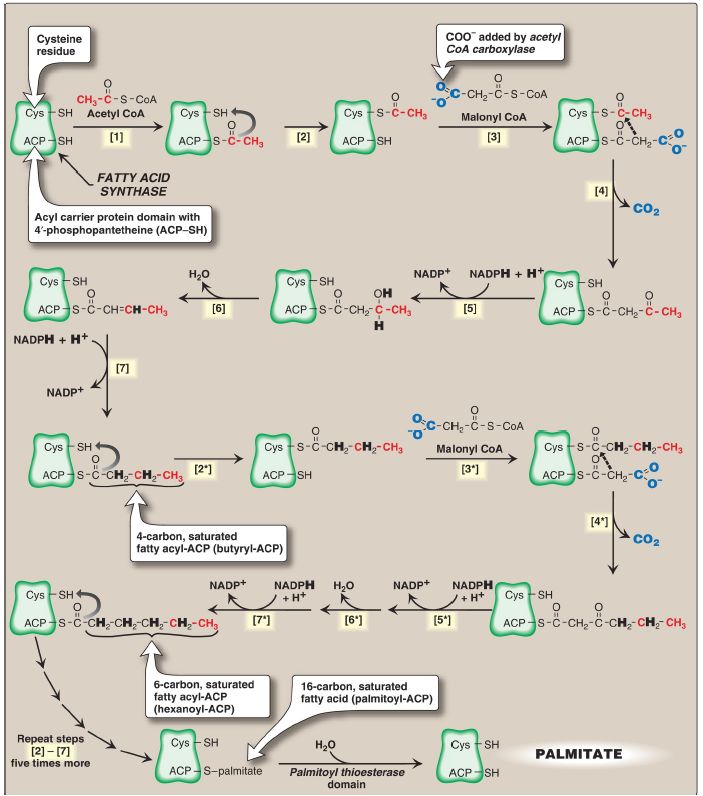
Figure 1: Synthesis of palmitate (16:0) by multifunctional fatty acid synthase. [Note: Numbers in brackets correspond to bracketed numbers in the text. A second repetition of the steps is indicated by numbers with an asterisk (*). Carbons provided directly by acetyl coenzyme A (CoA) are shown in red.] ACP = acyl carrier protein domain; CO2 = carbon dioxide; NADP(H) = nicotinamide adenine dinucleotide phosphate.
1. An acetyl group is transferred from acetyl CoA to the –SH group of the ACP. Domain: Malonyl/acetyl CoA–ACP transacylase.
2. Next, this two-carbon fragment is transferred to a temporary holding site, the –SH group of a cysteine residue on the condensing enzyme domain (see [4] below).
3. The now-vacant ACP accepts a three-carbon malonyl group from malonyl CoA. Domain: Malonyl/acetyl CoA–ACP transacylase.
4. The acetyl group on the cysteine residue condenses with the malonyl group on ACP as the CO2 originally added by ACC is released. The result is a four-carbon unit attached to the ACP domain. The loss of free energy from the decarboxylation drives the reaction. Domain: 3- Ketoacyl–ACP synthase, also known as condensing enzyme.
The next three reactions convert the 3-ketoacyl group to the corresponding saturated acyl group by a pair of NADPH-requiring reductions and a dehydration step.
1. The keto group is reduced to an alcohol. Domain: 3-Ketoacyl–ACP reductase.
2. A molecule of water is removed, creating a trans double bond between carbons 2 and 3 (the α- and β-carbons). Domain: 3-Hydroxyacyl–ACP dehydratase.
3. The double bond is reduced. Domain: Enoyl–ACP reductase.
This sequence of steps results in the production of a four-carbon group (butyryl) whose three terminal carbons are fully saturated and which remains attached to the ACP domain. The steps are repeated (indicated by an asterisk), beginning with the transfer of the butyryl unit from the ACP to the cysteine residue [2*], the attachment of a malonyl group to the ACP [3*], and the condensation of the two groups liberating CO2 [4*]. The carbonyl group at the β-carbon (carbon 3, the third carbon from the sulfur) is then reduced [5*], dehydrated [6*], and reduced [7*], generating hexanoyl-ACP. This cycle of reactions is repeated five more times, each time incorporating a two-carbon unit (derived from malonyl CoA) into the growing fatty acid chain at the carboxyl end. When the fatty acid reaches a length of 16 carbons, the synthetic process is terminated with palmitoyl-S-ACP. [Note: Shorter-length fatty acids are produced in the lactating mammary gland.] Palmitoyl thioesterase, the final catalytic activity of FAS, cleaves the thioester bond, releasing a fully saturated molecule of palmitate (16:0). [Note: All the carbons in palmitic acid have passed through malonyl CoA except the two donated by the original acetyl CoA (the first acyl acceptor), which are found at the methyl (ω) end of the fatty acid. This underscores the rate-limiting nature of the ACC reaction.]



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
عقد جلسة حوارية عن ضحايا جرائم التطرف ضمن فعاليات اليوم الثاني لمؤتمر ذاكرة الألم
|
|
|