


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 19-12-2015
Date: 27-2-2021
Date: 31-3-2021
|
Transgenesis by DNA Pronuclear Injection
Transgenesis by DNA pronuclear injection is the oldest and still the most widely used method of introducing foreign genes into the mammalian germline, subsequently to generate mammalian transgenic animals (including mice, rats and livestock). The basis of this technique, which was developed in the early 1980s by, amongst others, Gordon, Ruddle, Brinster, Palmiter and colleagues, is the microinjection of DNA of interest (the transgene) into the pronucleus of fertilised oocytes (single-cell embryos) leading to the ectopic expression (overexpression) of the transgene in the genome.
Generation of a Transgenic Mouse
This is a multi-step process (Figure .1) which begins with the construction of the transgene, involves collection of fertilised oocytes microinjection of the transgene DNA into the pronucleus of the oocytes, reimplantation of those eggs into a foster mother, identification of pups carrying the transgene and establishment of the transgenic line.
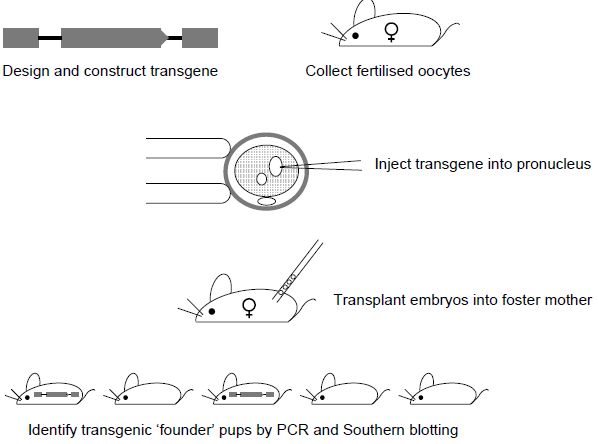
Figure 1. Generation of transgenic mice by DNA pronuclear injection. Fertilised oocytes are collected from superovulated females. Transgene DNA is injected into one of the pronuclei. Embryos are re-implanted into pseudo-pregnant foster females and the resulting pups are screened for integration of the transgene. Founder (transgene positive) mice are then mated with wild-type mice to test for germline transmission and transgene activity.
Step 1. Construction of the Transgene
The transgene can be any DNA sequence/gene of interest to be added to the mouse genome, i.e. it will be present in addition to the normal complement of mouse genes. It is possible to use overexpression of a transgene in several ways: (i) to determine the function of a gene or (ii) to
utilise its properties. For example, transgenes are often generated by linking a gene of interest with the regulatory regions of other known genes in order to express the gene of interest and its product at a higher level of expression or in a specific tissue or stage of development. Such experiments can be used to elucidate the normal function of the gene. For example, Figure .2 shows the transgene constructs used to overexpress the plasma membrane calcium pump isoform 4 (PMCA4) in the cardiomyocytes (under the regulation of the MLC2v promoter) and
in the vascular smooth muscle (driven by the SM22a promoter) – analysis of these transgenic lines has revealed that PMCA4 is involved in contractility in the heart and in the maintenance of peripheral blood pressure and vascular tone.
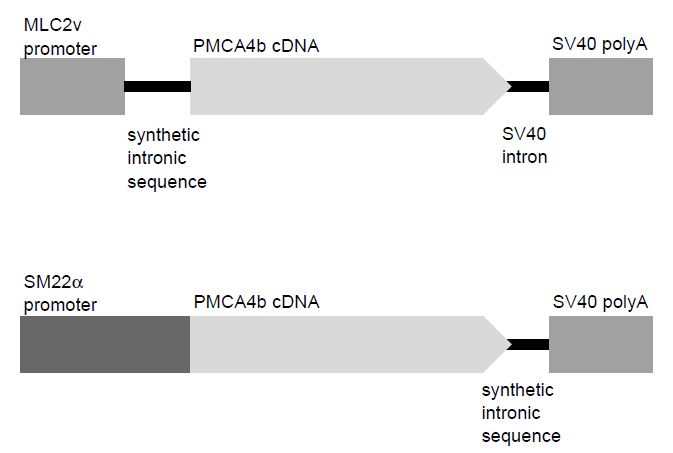
Figure .2 Construction of transgene. Examples of two transgenes designed to overexpress isoform 4 of the plasma membrane calcium pump (PMCA4b) in different tissues. Both constructs contain the same essential elements, i.e. a promoter sequence, cDNA of the gene of interest, a region of intronic sequence and a polyA tail. The top panel will result in the overexpression of PMCA4b in the heart, whereas the transgene in the bottom panel was designed to overexpress PMCA4b in vascular smooth muscle cells.
It is possible to use DNA pronuclear injection experiments to determine the function of regulatory elements of genes by fusing them to a reporter gene such as LacZ or GFP, whose expression can be readily detected.8,9 It is also possible to use a transgene to express the wild-type form of a gene in a mouse that carries an endogenous mutant form i.e. it can be used to rescue a genetic defect.Transgene design has a major influence on the successful expression of the microinjected DNA; there are a number of elements that must be incorporated into the transgene construct, in addition to the protein coding sequence of the gene of interest, that are critical for gene expression.
Essentially a transgene should also contain a promoter, an intron and a transcriptional stop sequence. The promoter is the regulatory sequence which determines in which cells and at what time the transgene is active and is usually derived from sequences upstream of, and including, the transcriptional start site which contain the necessary regulatory elements for transcription. The protein coding sequence is usually derived from the cDNA of the gene of interest and will contain both the ATG start codon and a translational stop codon. It is important to include an intron in the transgene construct as this increases transgene activity;11 however, this does not need to be an intron from the gene of interest. It is not clear why the inclusion of an intron has a positive effect on transgene expression, but it is hypothesised to be due to a functional link between transcription and splicing. The transgene must also contain a transcriptional stop signal including a polyA addition sequence (AAAUAA).
A number of transgenes have included both the intron and transcriptional stop signal at the end of the coding sequence (as in Figure .2: SM22a–PMCA4b transgene), although they can be included separately (see Figure .2: MLC2v–PMCA4b transgene).
It is clear that the construction of a transgene is a multi-step cloning process which is most conveniently carried out in a bacterial plasmid. It has been shown that it is important to remove all of the prokaryotic plasmid sequences from the transgene prior to microinjection as its inclusion may inhibit its expression. It is also clear that DNA that has been linearised prior tomicroinjection, rather than being left in its circular or supercoiled state, will integrate with greater efficiency. Interestingly, although a large transgene (430 kb) may present technical difficulties when cloning and isolating the DNA, their large size does not appear to effect the frequency of integration into the genome. The final aspect to consider is the purity of the DNA to be microinjected as it must be free of any contaminants which might be harmful to the oocytes to be injected.
Step 2. Collection of Fertilised Oocytes
To improve the efficiency of the generation of the transgenic mice, it is essential to have a large number of viable fertilised eggs (oocytes) available for microinjection. Normally female mice will generate 6–10 eggs when naturally ovulating; however, it is possible to recover 20–30 eggs from a single female by inducing superovulation. The consequence is that less female mice are required for the production of a suitable number of quality eggs. Young female mice are injected with a combination of hormones, which mimic natural mouse hormones, and these
ensure that large numbers of mature eggs are released from the ovaries simultaneously; treatment with follicle stimulating hormone is followed 44–48 h later by treatment with leutinising hormone. Fertilised oocytes are collected from the oviducts of the females following overnight mating with stud males. The oocytes will be collected at 0.5 days post coitum (dpc) as this allows time for the sperm to complete fertilisation following mating. In addition, at this stage the pronuclei from both gametes will be visible for several hours after embryo collection, which is necessary to allow successful injection of the transgene DNA.
Step 3. Pronuclear Injection of DNA Transgene
Microinjection of the transgene requires specialist, expensive equipment and highly trained personnel. The whole microinjection process is visualised under an inverted microscope which is attached to two micromanipulators, one to manipulate the glass pipette used to hold and secure the oocyte during the injection process (known as the holding pipette) and the other to control the fine glass pipette used to inject the DNA transgene into the pronucleus (Figure .3). For an excellent and detailed technical description of the microinjection process, see Hogan et al.

Figure .3 Microinjection of DNA into the pronucleus. The oocyte is secured by gentle suction on to the holding pipette. The microinjection pipette is advanced through the zona pellucida, it pierces the plasma membrane and is then pushed into the pronucleus. The oocyte has two pronuclei, either of which can be injected; the male pronucleus tends to be the largest and therefore easiest to visualise and inject. The DNA is then expelled from the injection pipette causing the pronucleus to swell.
It is usual that between 60 and 80% of the microinjected embryos will survive the process (viability can be tested by culturing the embryos to the two-cell stage) and be suitable for re-implantation into a surrogate female. Surrogate/recipient females are rendered pseudo-pregnant by mating with either a vasectomised or genetically sterile male. Of these re-implanted embryos, between 10 and 25%will survive to term. It is this high attrition rate of embryos at each stage which necessitates the use of such large numbers of fertilised eggs at the beginning of the whole procedure.The transgene will integrate randomly into the genome, into any chromosome, including the sex chromosomes and often in multiple copies in a head-to-tail array. Interestingly, it appears that integration most often occurs at just one site in the genome, even if multiple copies integrate.
Experience has shown that when microinjecting linearised DNA, approximately 25% of the resulting pups carry the transgene, these are known as founder transgenics. Due to the precise timing of injection and therefore integration of the transgene into the pronucleus, prior to replication, a high proportion (approximately 70%) of the transgenic mice will carry the transgene in every cell of the body. It is essential that the transgene integrates into the germ cells to be able to transmit the gene modification to the next generation. In those mice in which integration of the transgene occurred after DNA replication, the transgene will integrate into a proportion of the somatic and germ cells only; these mice are known as mosaics.
Step 4. Generation of a Transgenic Line
Founder transgenic mice are identified by DNA analysis including PCR and Southern blotting. It is common for DNA to be isolated from small pieces of tissue taken from the ears of the mice or from a small section of the end of the tail. The presence or absence of the transgene can be identified by PCR analysis using transgene-specific primers which will not amplify the endogenous gene locus. Southern blot analysis is then used to verify the integration of the transgene and to identify the number of copies that have inserted into the genome.
Once founders have been identified, they must be bred to wild-type mice to determine if the transgene can be passed to the next generation; in other words, they will be tested for germline transmission. The resultant pups must then be screened by PCR to identify heterozygous (Tg/+) transgenic mice from their wild-type (+/+) littermates – these would be expected in a 50:50 ratio.
One of the main drawbacks of generating transgenic mice by pronuclear injection of DNA is the so called position effect, i.e. the transgene can integrate at any chromosomal location and the site of integration itself could effect its expression. For example, a transgene could insert into a region of the chromosome that suppresses gene activity or could even insert into a functioning endogenous gene. Both of these scenarios would lead to a phenotype that is not solely due to the effect of the transgene. It is therefore essential that the phenotype of at least three independent founder lines carrying the same transgene are analysed to determine the effect of overexpression of the gene of interest.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|