


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 12-12-2016
Date: 24-10-2020
Date: 17-11-2020
|
Electron Volt as a Unit of Energy
In discussing the motion of an electron in an atom, quantities like meters and kilograms and joules are awkwardly large. There is, however, a unit of energy that is particularly convenient for discussing many applications, including the motion of electrons in atoms. This unit of energy, called the electron volt (abbreviated eV), is the amount of energy an electron would gain if it hopped from the negative to the positive terminal of a 1 volt battery. The numerical value is
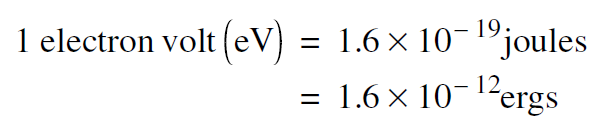 .........(1)
.........(1)
As an example, an electron in a cold (unexcited) hydrogen atom has a total energy of –13.6 eV. The fact that the electron's energy is negative means that the electron is bound to the proton–cannot escape.
The value –13.6 eV means that, in order to pull the electron out of the hydrogen atom, we would have to supply 13.6 eV of energy. In other words, the binding energy of the electron in a cold hydrogen atom is 13.6 eV. The number is 13.6 eV is much easier to discuss and remember than 2.1 6 × 10 – 18joules .
Another unit that is convenient for discussing atoms is the angstrom (abbreviated A° ) which is 10 – 8cm or 10 – 10 meters.
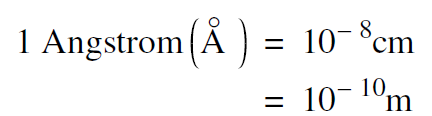 ......(2)
......(2)
A hydrogen atom has a diameter of 1 A° and all atoms are approximately the same size. Even the largest atom, Uranium, has a diameter of only a few angstroms. In the hydrogen molecule ion, the separation of the protons is 1.07 A° .



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|