


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 15-1-2021
Date: 22-12-2020
Date: 11-12-2020
|
Introduction to Drug Discovery
1. Basics of Drug Discovery in the Biopharmaceutical Industry
Historically, pharmaceutical products have been developed primarily through the random testing of thousands of synthetic compounds and natural products. The traditional approach to drug discovery is based on the generation of a hypothesis based on biochemistry and a pharmacological approach to a disease. Targets are defined on the basis of this hypothesis and lead discovery is a matter of chance. The classical drug discovery effort also involves isolating and characterising natural products with some biological activity. These compounds are then ‘refined’ by redesigning their molecular structure to yield new entities with higher biological activity and lower toxicity/side-effects. The main limitation of such a process is that the discovery of natural products with defined biological activity is essentially a hit-or-miss approach and therefore lacks a rational basis.
2. Historical Landmarks in Drug Discovery and Development
Drug discovery is as old as human history. Ancient human cultures had medicines for illness that were discovered by trial and error from plant, animal and mineral sources. The effective constituents of some of the herbal medicines have been isolated and form the basis of some modern medicines. Modern medicine is considered to have started in the nineteenth century, although several important discoveries, notably smallpox vaccine, were made close to the end of the eighteenth century.
Modern pharmaceuticals and drug discovery started to develop in the twentieth century. Five stages in the evolution of drug development are shown in Table 1.
Table 1 Five stages in the evolution of drug development.
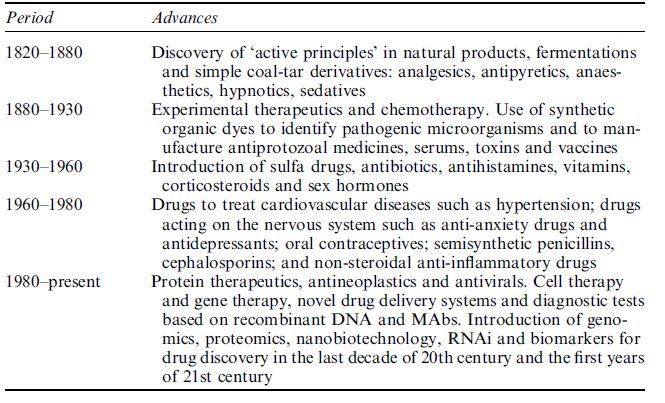
In the earlier part of the twentieth century, drug discovery was based on chemistry and serendipity played a role. For the first generation of pharmaceutical products, studies were done in vivo; there were no clinical trials. Most of the drugs were used for palliation or symptomatic relief in a medical environment that was more art than science. Pioneer pharmaceutical companies were still growing.
The second generation of pharmaceutical products, which were based on biology, started to be introduced around the middle of the twentieth century. There was still a considerable amount of empiricism and in vitro methods started to develop for the study of drugs. The medicines were more effective and could modify the disease process. Laboratory methods started to be used increasingly to support clinical diagnosis in an environment of experience-based medicine, which was still a mixture of art and science. The last quarter of the century saw the development of biotechnology.
Towards the end of the twentieth century and in the twenty-first century, genomics and genetics started to play an important role in drug development, with a marked increase in the number of biotechnologybased drugs. Drug discovery activities have accelerated with the use of high-throughput methods, biochips and robotics. The role of chemistry is still considered to be important and proteomics is gradually assuming an important role in drug discovery in the post-genomic era. Bioinformatics and in silico methods are being used for drug discovery. The clinical environment is evidence-based medicine with the start of evolution of personalised medicines. With all the scientific advances in medicine, there is still some resurgence of alternative or complementary medicine and an evaluation of drugs from natural sources within the pharmaceutical industry.
3. Current Status of Drug Discovery
The drug discovery process is shown in Figure 1. The usual duration of this process is 5–6 years, which is half of the total development time (10–12 years) taken from target identification to marketing of a drug.
Traditional screening methods are slow and labour intensive and have limited the number and chemical diversity of the compounds and targets that can be tested in a given assay. Even though many thousands of distinct chemical structures exist, it is not unusual for screening utilising this approach to be terminated at the end of several years with no lead compounds identified, having examined only a small fraction of available compounds. This limitation of speed and scale often restricts both the quality and quantity of lead compounds available for further testing and development, thereby hindering drug discovery.
Several ‘hits’ are produced as a result of high-throughput screening (HTS). The hit-to-lead stage has been added to the drug discovery. Multiple parameters are optimised in parallel with produce leads with a balanced profile of biological and physicochemical properties. New
technologies are playing an increasing role in this process. It is desirable to have multiple series in hit optimisation so that more than one series is available for lead optimisation.
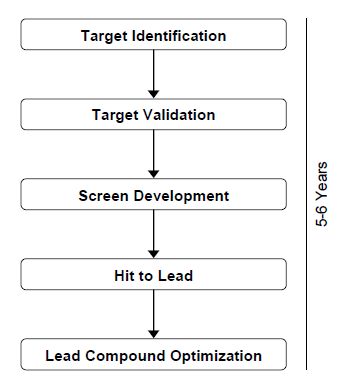
Figure .1 Drug discovery process.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|