


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 17-9-2020
Date: 31-12-2020
Date: 17-9-2020
|
The electron
An electron has exactly the same charge quantity as a proton but with opposite polarity. Electrons are far less massive than protons, however. It would take about 2,000 electrons to have the same mass as a single proton.
One of the earliest theories concerning the structure of the atom pictured the electrons embedded in the nucleus like raisins in a cake. Later, the electrons were imagined as orbiting the nucleus, making every atom like a miniature star system with the electrons as the planets. Still later, this view was modified further. In today’s model of the atom, the electrons are fast-moving, and they describe patterns so complex that it is impossible to pinpoint any individual particle at any given instant of time. All that can be done is to say that an electron just as likely will be inside a certain sphere as outside. These spheres are known as electron shells. The centers of the shells correspond to the position of the atomic nucleus. The greater a shell’s radius, the more energy the electron has. Figure 1 is a greatly simplified drawing of what happens when an electron gains just enough energy to “jump” from one shell to another shell representing more energy.
Electrons can move rather easily from one atom to another in some materials. These substances are electrical conductors. In other substances, it is difficult to get electrons to move. These are called electrical insulators. In any case, however, it is far easier to move electrons than it is to move protons. Electricity almost always results, in some way, from the motions of electrons in a material.
Generally, the number of electrons in an atom is the same as the number of protons. The negative charges therefore exactly cancel out the positive ones, and the atom is electrically neutral. Under some conditions, however, there can be an excess or shortage of electrons. High levels of radiant energy, extreme heat, or the presence of an electrical field (to be discussed later) can “knock” electrons loose from atoms, upsetting the balance.
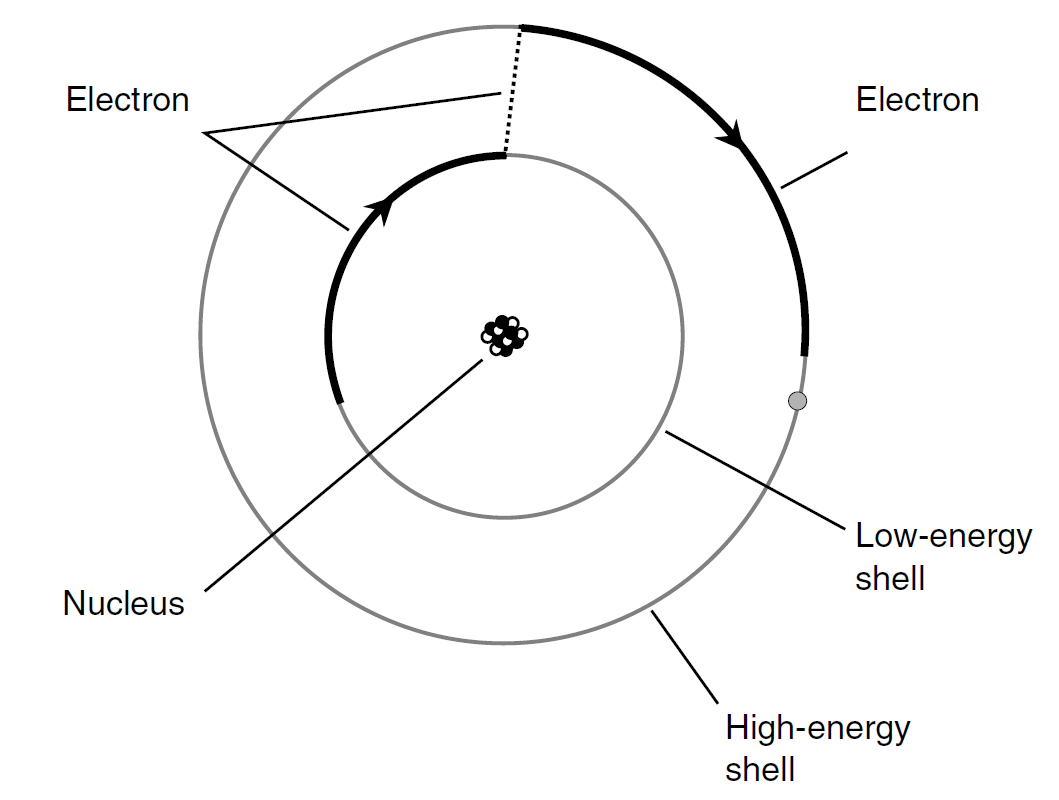
Fig. 1. Electrons exist at defined levels, each level corresponding to a specific, fixed energy state.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|