


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 7-2-2016
Date: 5-8-2018
Date: 27-9-2019
|
The functional groups that undergo nucleophilic acyl substitutions are called carboxylic acid derivatives: these include carboxylic acids themselves, carboxylates (deprotonated carboxylic acids), amides, esters, thioesters, and acyl phosphates. Two more examples of carboxylic acid derivatives which are less biologically relevant but important in laboratory synthesis are carboxylic acid anyhydrides and acid chlorides.
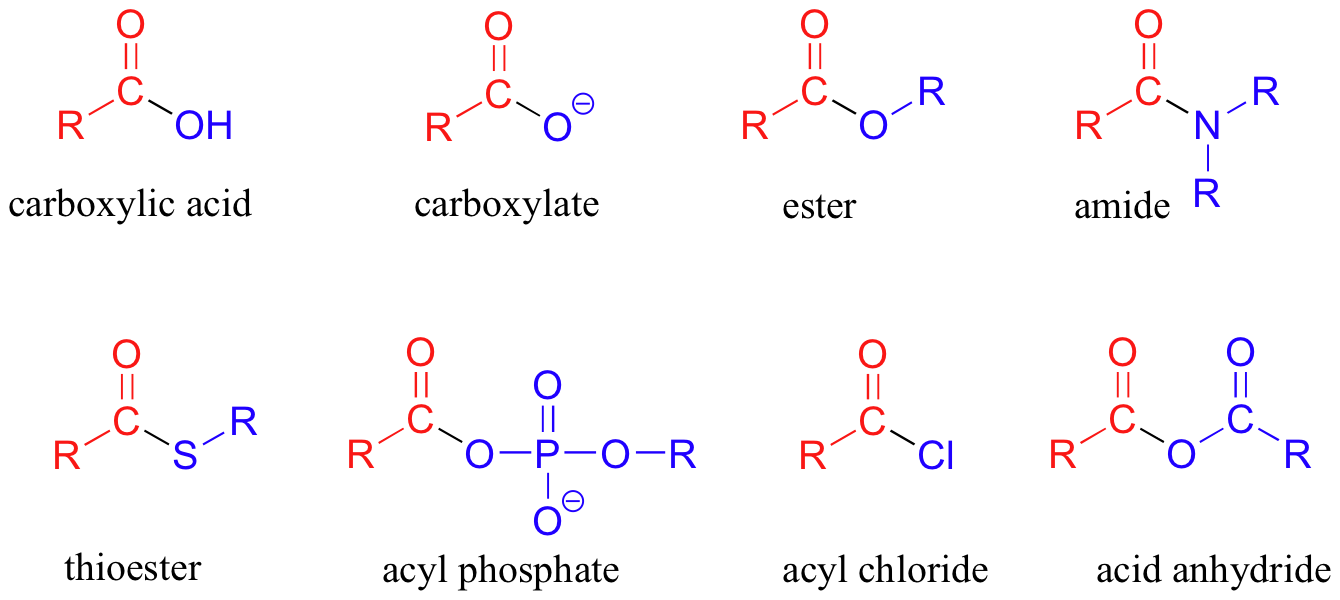
The carboxylic acid derivatives can be distinguished from aldehydes and ketones by the presence of a group containing an electronegative heteroatom - usually oxygen, nitrogen, or sulfur – bonded directly to the carbonyl carbon. You can think of a carboxylic acid derivative as having two sides. One side is the carbonyl group and the attached alkyl group: this is called an acyl group (in the specific case where R is a methyl group, the term acetyl group is used). One the other side is the heteroatom-containing group: in this text, we will sometimes refer to this component as the ‘acyl X' group (this, however, is not a standard term in organic chemistry).
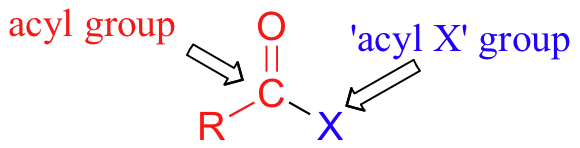
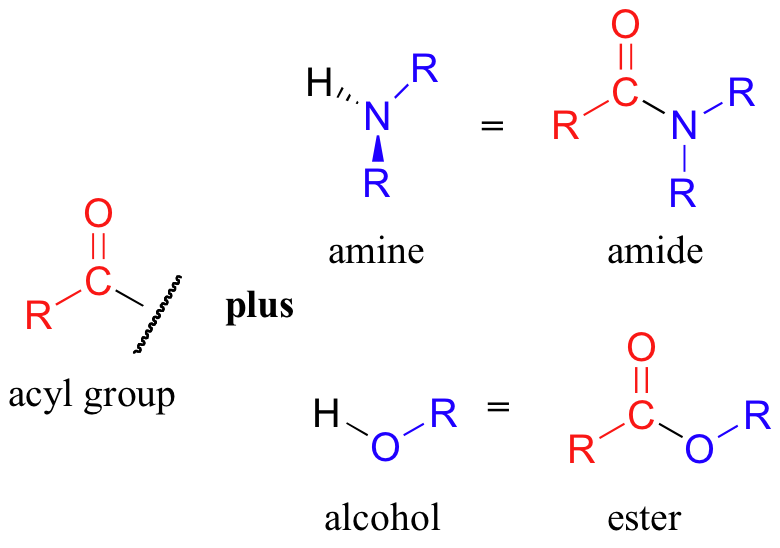
Notice that the acyl X groups are simply deprotonated forms of other functional groups: in an amide, for example, the acyl X group is an amine, while in an ester the acyl X group is an alcohol.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
العتبة العباسية المقدسة تطلق النسخة الحادية عشرة من مسابقة الجود العالمية للقصيدة العمودية
|
|
|