


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 27-8-2019
Date: 24-5-2017
Date: 17-9-2019
|
The presence of that hydrogen atom makes aldehydes very easy to oxidize. Or, put another way, they are strong reducing agents. The most common reagent for this conversion is CrO3 in aqueous acid. This reaction generally gives good yields at room temperature.
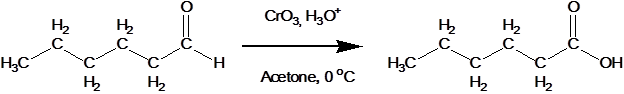
Unfortunately, the acid condition for the previous reaction can cause unwanted side reaction. If this problem occurs it can be rectified by using a solution of sliver oxide, Ag2O, in aqueous ammonia, also called Tollens' reagent.
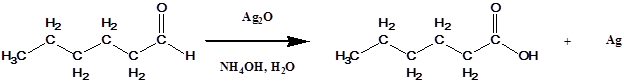
Because ketones do not have that particular hydrogen atom, they are resistant to oxidation, and only very strong oxidizing agents like potassium manganate(VII) solution (potassium permanganate solution) oxidize ketones. However, they do it in a destructive way, breaking carbon-carbon bonds and forming two carboxylic acids.




|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|