
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 9-4-2017
Date: 14-11-2019
Date: 25-11-2019
|
Hydrogen bonding occurs between molecules in which a hydrogen atom is attached to a strongly electronegative element: fluorine, oxygen or nitrogen. In the case of alcohols, hydrogen bonds occur between the partially-positive hydrogen atoms and lone pairs on oxygen atoms of other molecules.
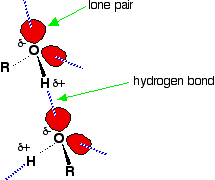
The hydrogen atoms are slightly positive because the bonding electrons are pulled toward the very electronegative oxygen atoms. In alkanes, the only intermolecular forces are van der Waals dispersion forces. Hydrogen bonds are much stronger than these, and therefore it takes more energy to separate alcohol molecules than it does to separate alkane molecules. This the main reason for higher boiling points in alcohols.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|