


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-9-2018
Date: 25-9-2020
Date: 7-10-2020
|
We noted earlier that addition reactions of alkenes often exhibited stereoselectivity, in that the reagent elements in some cases added syn and in other cases anti to the the plane of the double bond. Both reactants in the Diels-Alder reaction may demonstrate stereoisomerism, and when they do it is found that the relative configurations of the reactants are preserved in the product (the adduct). The following drawing illustrates this fact for the reaction of 1,3-butadiene with (E)-dicyanoethene. The trans relationship of the cyano groups in the dienophile is preserved in the six-membered ring of the adduct. Likewise, if the terminal carbons of the diene bear substituents, their relative configuration will be retained in the adduct. Using the earlier terminology, we could say that bonding to both the diene and the dienophile is syn. An alternative description, however, refers to the planar nature of both reactants and terms the bonding in each case to be suprafacial (i.e. to or from the same face of each plane). This stereospecificity also confirms the synchronous nature of the 1,4-bonding that takes place.
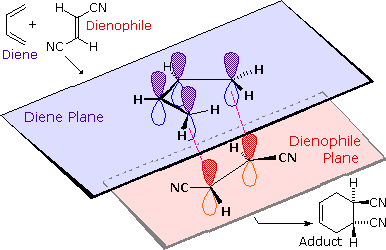
The essential characteristics of the Diels-Alder cycloaddition reaction may be summarized as follows:
These features are illustrated by the following eight examples, one of which does not give a Diels-Alder cycloaddition.
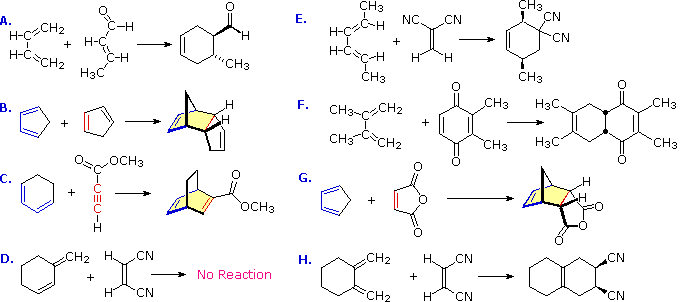
There is no reaction in example D because this diene cannot adopt an s-cis orientation. In examples B, C, F, G & H at least one of the reactants is cyclic so that the product has more than one ring, but the newly formed ring is always six-membered. In example B the the same cyclic compound acts as both the diene colored blue) and the dienophile (colored red). The adduct has three rings, two of which are the five-membered rings present in the reactant, and the third is the new six-membered ring (shaded light yellow). Example C has an alkyne as a dienophile (colored red), so the adduct retains a double bond at that location. This double bond could still serve as a dienophile, but in the present case the diene is sufficiently hindered to retard a second cycloaddition. The quinone dienophile in reaction F has two dienophilic double bonds. However, the double bond with two methyl substituents is less reactive than the unsubstituted dienophile due in part to the electron donating properties of the methyl groups and in part to steric hindrance. The stereospecificity of the Diels-Alder reaction is demonstrated by examples A, E & H. In A & H the stereogenic centers lie on the dienophile, whereas in E these centers are on the diene. In all cases the configuration of the reactant is preserved in the adduct.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|