
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-12-2018
Date: 13-11-2018
Date: 20-12-2018
|
In chlorine(VII) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. This produces a much bigger molecule, and so you would expect its melting point and boiling point to be higher than chlorine(I) oxide. Chlorine(VII) oxide is a colourless oily liquid at room temperature. In the diagram, for simplicity I have drawn a standard structural formula. In fact, the shape is tetrahedral around both chlorines, and V-shaped around the central oxygen.
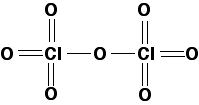



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|