


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-10-2018
Date: 20-12-2018
Date: 22-1-2019
|
Silanes are silicon-hydrogen compounds. Carbon-hydrogen compounds form the backbone of the living world with seemingly endless chains of hydrocarbons. With the same valence configuration, and thus the same chemical versatility, silicon could conceivably play a role of similar organic importance. But silicon does not play an integral role in our day to day biology. One principal reasons underlies this.
Like hydrocarbons, silanes progressively grow in size as additional silicon atoms are added. But there is a very quick end to this trend. The largest silane has a maximum of six silicon atoms. (see Figure1)
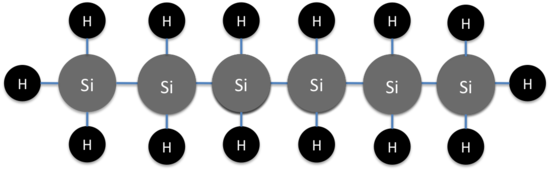
Figure 1: The largest silane, hexasilane
Hexasilane is the largest possible silane because Si-Si bonds are not particularly strong. In fact, silanes are rather prone to decomposition. Silanes are particularly prone to decomposition via oxygen. Silanes also have a tendency to swap out there hydrogens for other elements and become organosilanes. (see Figure 2)
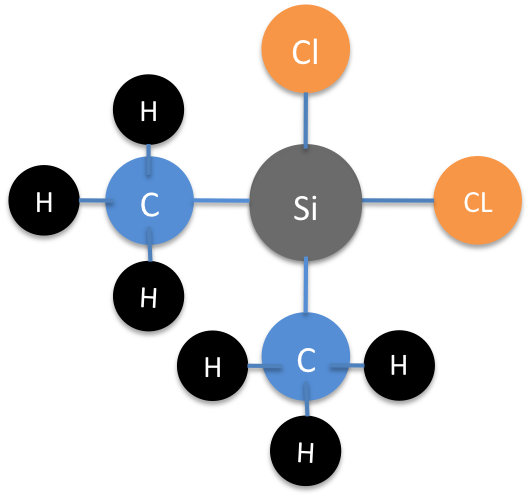
Figure 2: The organosilane, dichlorodimethylsilane
Silanes have a variety of industrial and medical uses. Among other things, silanes are used as water repellents and sealants.



|
|
|
|
الصين.. طريقة لمنع تطور قصر النظر لدى تلاميذ المدارس
|
|
|
|
|
|
|
ماذا سيحدث خلال كسوف الشمس يوم السبت؟
|
|
|
|
|
|
|
بمشاركة مجتمعيّة واسعة .. اختتام فعاليات المجلس العلوي الثقافي السنوي الثاني
|
|
|