


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-8-2018
Date: 17-8-2018
Date: 17-8-2018
|
The common feature of these lipids is that they are all esters of moderate to long chain fatty acids. Acid or base-catalyzed hydrolysis yields the component fatty acid, some examples of which are given in the following table, together with the alcohol component of the lipid. These long-chain carboxylic acids are generally referred to by their common names, which in most cases reflect their sources. Natural fatty acids may be saturated or unsaturated, and as the following data indicate, the saturated acids have higher melting points than unsaturated acids of corresponding size. The double bonds in the unsaturated compounds listed on the right are all cis (or Z).
Unsaturated |
||
|---|---|---|
|
Formula |
Common Name |
Melting Point |
| CH3(CH2)5CH=CH(CH2)7CO2H | palmitoleic acid | 0 ºC |
| CH3(CH2)7CH=CH(CH2)7CO2H | oleic acid | 13 ºC |
| CH3(CH2)4CH=CHCH2CH=CH(CH2)7CO2H | linoleic acid | -5 ºC |
| CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7CO2H | linolenic acid | -11 ºC |
|
CH3(CH2)4(CH=CHCH2)4(CH2)2CO2H |
arachidonic acid | -49 ºC |
Saturated |
||
|---|---|---|
|
Formula |
Common Name |
Melting Point |
| CH3(CH2)10CO2H | lauric acid | 45 ºC |
| CH3(CH2)12CO2H | myristic acid | 55 ºC |
| CH3(CH2)14CO2H | palmitic acid | 63 ºC |
| CH3(CH2)16CO2H | stearic acid | 69 ºC |
| CH3(CH2)18CO2H | arachidic acid | 76 ºC |
The higher melting points of the saturated fatty acids reflect the uniform rod-like shape of their molecules. The cis-double bond(s) in the unsaturated fatty acids introduce a kink in their shape, which makes it more difficult to pack their molecules together in a stable repeating array or crystalline lattice. The trans-double bond isomer of oleic acid, known as elaidic acid, has a linear shape and a melting point of 45 ºC (32 ºC higher than its cis isomer). The shapes of stearic and oleic acids are displayed in the models below.
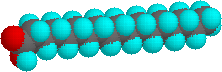 |
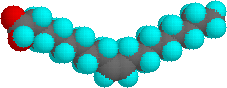 |
|---|---|
|
stearic acid |
oleic acid |
Two polyunsaturated fatty acids, linoleic and linolenic, are designated "essential" because their absence in the human diet has been associated with health problems, such as scaley skin, stunted growth and increased dehydration. These acids are also precursors to the prostaglandins, a family of physiologically potent lipids present in minute amounts in most body tissues.
Because of their enhanced acidity, carboxylic acids react with bases to form ionic salts, as shown in the following equations. In the case of alkali metal hydroxides and simple amines (or ammonia) the resulting salts have pronounced ionic character and are usually soluble in water. Heavy metals such as silver, mercury and lead form salts having more covalent character (3rd example), and the water solubility is reduced, especially for acids composed of four or more carbon atoms.
| RCO2H | + | NaHCO3 |  |
RCO2(–) Na(+) + CO2 + H2O |
| RCO2H | + | (CH3)3N: |  |
RCO2(–) (CH3)3NH(+) |
| RCO2H | + | AgOH |  |
RCO2δ(–) Agδ(+) + H2O |



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|