


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 1-10-2019
Date: 2-10-2018
Date: 8-7-2018
|
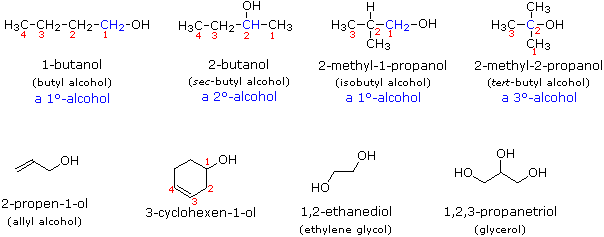
Alcohol Nomenclature
In the IUPAC system of nomenclature, functional groups are normally designated in one of two ways. The presence of the function may be indicated by acharacteristic suffix and a location number. This is common for the carbon-carbon double and triple bonds which have the respective suffixes ene and yne. Halogens, on the other hand, do not have a suffix and are named as substituents, for example: (CH3)2C=CHCHClCH3 is 4-chloro-2-methyl-2-pentene. If you are uncertain about the IUPAC rules for nomenclature you should review them now.
Alcohols are usually named by the first procedure and are designated by an ol suffix, as in ethanol, CH3CH2OH (note that a locator number is not needed on a two-carbon chain). On longer chains the location of the hydroxyl group determines chain numbering. For example: (CH3)2C=CHCH(OH)CH3 is 4-methyl-3-penten-2-ol. Other examples of IUPAC nomenclature are shown below, together with the common names often used for some of the simpler compounds. For the mono-functional alcohols, this common system consists of naming the alkyl group followed by the word alcohol. Alcohols may also be classified as primary, 1º, secondary, 2º & tertiary, 3º, in the same manner as alkyl halides. This terminology refers to alkyl substitution of the carbon atom bearing the hydroxyl group (colored blue in the illustration).
Many functional groups have a characteristic suffix designator, and only one such suffix (other than "ene" and "yne") may be used in a name. When the hydroxyl functional group is present together with a function of higher nomenclature priority, it must be cited and located by the prefix hydroxy and an appropriate number. For example, lactic acid has the IUPAC name 2-hydroxypropanoic acid.
Compounds incorporating a C–S–H functional group are named thiols or mercaptans. The IUPAC name of (CH3)3C–SH is 2-methyl-2-propanethiol, commonly called tert-butyl mercaptan. The chemistry of thiols will not be described here, other than to note that they are stronger acids and more powerful nucleophiles than alcohols.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|