

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The hydrogen cycle
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
768
2025-10-26
365
The hydrogen cycle
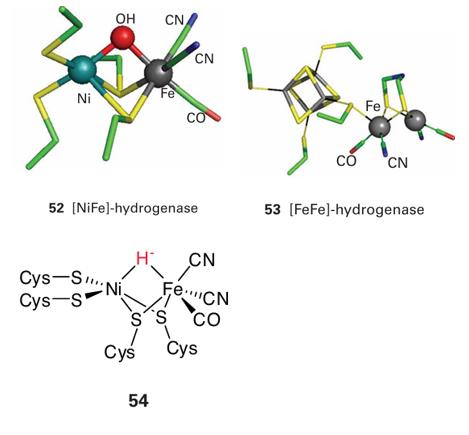
Key point: The active sites of hydrogenases contain Fe or Ni, along with CO and CN ligands. It has been estimated that 99 per cent of all organisms utilize H2. Even if these species are almost entirely microbes, the fact remains that almost all bacteria and archaea possess extremely active metalloenzymes, known as hydrogenases, that catalyse the interconversion of H2 and H (as water). The elusive molecule H2 is produced by some organisms (it is a waste product) and used by others as a fuel, helping to explain why so little H2 is in fact detected in the atmosphere (Box 10.1). Human breath contains measurable amounts of H2 due to the action of bacteria in the gut. Hydrogenases are very active enzymes, with turnover frequencies (molecules of substrate transformed per second per molecule of enzyme) exceeding 10 000 s 1. They are therefore attracting much attention for the insight they can provide regarding clean production of H2 (Section 10.4, Box 10.3) and oxidation of H2 in fuel cells—technology that currently depends greatly on Pt (Box 5.1). There are three classes of hydrogenase, based on the structure of the active site. All contain Fe and some also contain Ni. The two best-characterized types are known as [NiFe]-hydrogenases and [FeFe]-hydrogenases, and structures of the active sites of two representative enzymes are shown as (52) and (53). The active sites contain at least one CO ligand, and provide further examples of biological organometallic active sites (in addition to coenzyme B12). Further ligation is provided by CN, cysteine (and sometimes selenocysteine), and the active site of [FeFe]-hydrogenases (the site is commonly referred to as an ‘H-cluster’) contains a [4Fe-4S] cluster linked by a bridging cysteine thiolate, and an unusual bridging bidentate ligand that is thought to be either dithiomethylamine or dithiomethylether. These fragile active sites are buried deeply within the enzyme, thus necessitating special pores and pathways to convey H2 and H, and a relay of FeS clusters for long-range electron transfer (as shown in Fig. 27.26). The [FeFe]-enzymes tend to operate in the direction of H2 production; they are usually found in strictly anaerobic organisms and are very sensitive to O2. The mechanism of catalysis is uncertain, but it involves the participation of unusual Fe(I) species: the form assigned as Fe(I)Fe(I) binds H and that assigned as Fe (II)Fe(I) binds H2, probably in an analogous mannerto the dihydrogen complex shown (11) in Section 10.6. The [NiFe]-enzymes are noted for H2 oxidation and a catalytically active form assigned by EPR spectroscopy as Ni (III)–H (a hydrido complex) has been identified (54). Single-crystal EPR studies show that the unpaired electron is coupled strongly to an H-atom nucleus lying along an axis that points towards the Fe (that is, the H is in a bridging position). The active sites of [NiFe]- and [FeFe]-hydrogenases react with O2, often irreversibly, and this sensitivity is a limiting fac tor in developing and exploiting renewable H2 production by microorganisms (Section 10.4 and Box 10.1).
 الاكثر قراءة في اختراعات ومكتشفون
الاكثر قراءة في اختراعات ومكتشفون
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















