

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Iron-transport proteins in higher organisms
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص736-737
2025-10-22
445
Iron-transport proteins in higher organisms
There are several important, structurally similar Fe-transport proteins known collectively as transferrins. The best characterized examples are serum transferrin (in blood plasma), ovotransferrin (in egg-white), and lactoferrin (in milk). The apoproteins are potent anti-bacterial agents as they deprive microbes of their iron. Transferrins are also present in tears, serving to cleanse eyes after irritation. All these transferrins are glycoproteins (pro-tein molecules modified by covalently bound carbohydrate) with molar masses of about 80 kg mol-1 and containing two separated and essentially equivalent binding sites for Fe. Complexation of Fe (III) at each site involves simultaneous binding of HCO3 or CO32- and release of H:
apo-TF + Fe (III) + HCO3– → TF–Fe (III)–CO32– +H+
where TF denotes transferrin. For each site, the association constant under physiological conditions (pH=7) is in the range 1022–1026. However, its value depends strongly on the pH and this dependence is the main factor controlling Fe uptake and release. Transferrin consists of two very similar parts, termed the N-lobe and the C-lobe (Fig. 27.12). The protein is a product of gene duplication because the structure of the first half of the molecule can almost be overlaid on the second half. Each half consists of two domains, 1 and 2, which together form a cleft with a binding site for Fe (III). There is a considerable proportion of α helix, resulting in flexibility. Complexation with Fe (III) causes a conformational change consisting of a hinge motion involving domains 1 and 2 at each lobe. Binding of Fe (III) causes the domains to come together. In each active site (19), a single Fe atom is coordinated by widely dispersed amino acid side chains from both domains and the connecting region, hence the change in conformation that occurs. The protein ligands are carboxylate-O (Asp), two phenolate-O (Tyr), and an imidazole-N (His). Only one of the aspartate carboxylate-O atoms is coordinated. The protein ligands form part of a distorted octahedral coordination sphere. The coordination is completed by bidentate binding to the exogenous carbonate, which is referred to as a synergistic ligand because Fe binding depends on its presence. In certain cases phosphate is bound instead of carbonate. As expected from the predominantly anionic ligand set, Fe (III) binds much more tightly than Fe (II). However, ions similar to Fe (III), particularly Ga (III) and Al(III), also bind tightly, so that these metals can use the same transport system to gain access to tissues.
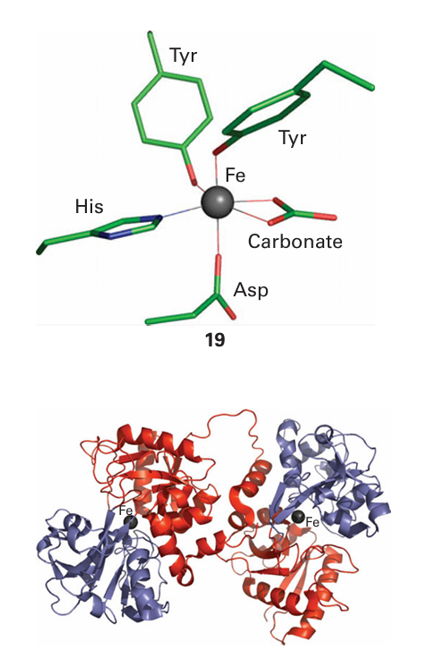
Figure 27.12 Structure of the Fe-transport protein transferrin: the identical halves of the molecule each coordinate to a single Fe(III) atom (the black spheres) between two lobes. This coordination causes a conformational change that allows transferrin to be recognized by the transferrin receptor.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















