
Sodium and potassium transport
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص731-733
الجزء والصفحة:
ص731-733
 2025-10-22
2025-10-22
 72
72
Sodium and potassium transport
Key points: Transport across a membrane is active (energized) or passive (spontaneous); the flow of ions is achieved by proteins known as ion pumps (active) and channels (passive). In Chapter 11 we saw that differentiating between Na and K, two ions that are very similar except for their radii (Resource section 1), is achieved through their selective complexation by special ligands, such as crown ethers and cryptands, with dimensions ap propriate for coordination to one particular kind of ion. Organisms use this principle in the molecules known as ionophores, which have hydrophobic exteriors that make them soluble in lipids. The antibiotic valinomycin (Section 11.16) is an ionophore that has a high selectivity for K, which is coordinated by six carbonyl groups. It enables K to pass through a bacterial cell membrane and thereby dissipate the electrical potential difference, so causing the bacterium’s death. At the higher end of the complexity scale are the ion channels, which are large mem brane-spanning proteins that allow selective transport of K+ and Na+ (as well as Ca2+ and Cl-) and are responsible for electrical conduction in nervous systems as well as in coupled transport of solutes.4 Figure 27.4 shows the important structural aspects of the potential gated K channel. Moving from the inside surface of the membrane, the enzyme has a pore (which can open and close on receipt of a signal) leading into a central cavity about 1 nm in diameter; up to this stage K ions can remain hydrated. Polypeptide helices pointing at this cavity have their partial charges directed in such a way as to favour population by cations, resulting in a local K concentration of approximately 2 m. Above the central cavity the tunnel contracts into a selectivity filter consisting of helical ladders of closely spaced peptide carbonyl-O donors that form a sequence of four cubic eightfold coordination sites. During operation of the channel these sites are occupied, at any one time, by a queue of two K ions and two H2 O molecules in alternate fashion, as in …K+…H2O…K+…H2O…. The rate of passage of K ions through the selectivity filter is close to the limit for diffusion control. A plausible mechanism for selective K transport (Fig. 27.5) involves concerted displacement of K ions between adjacent cubic carbonyl-O sites through intermediate, unstable octahedral states in which the K ions are coordinated equatorially by four carb onyl-O donors and axially by the two intervening H2O molecules. This mechanism is not
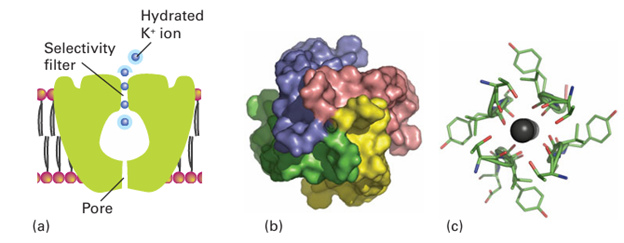
Figure 27.4 (a) Schematic structure of the K channel showing the different components and the transport of K ions: the blue halo represents hydration. (b) View of the enzyme from inside the cell showing the entrance pore that admits hydrated ions. (c) View looking up the selectivity filter showing how mobile dehydrated K ions are coordinated by peptide carbonyl-O atoms provided by each of the four subunits. Note the almost fourfold symmetry axis.
effective for Na because the cavity is too large, which accounts for the 104-fold selectivity of the channel for K+ over Na+. The binding is weak and fast because it is important to convey but not to trap K. Overall, the K channel acts by the mechanism illustrated in Fig. 27.6. Charged groups on the molecule move in response to a change in the membrane potential and cause the intracellular pore to open, so allowing entry of hydrated K ions. Selective binding of dehydrated K ions occurs in the filter region, a potential drop across the membrane is sensed, and the cavity closes. At this point the filter opens up to the external surface, where the K concentration is low and the K ions are hydrated and released. This release causes the protein to switch back to the original conformation and K ions again enter the filter. The Na /K pump (Na /K-ATPase), the enzyme that maintains the concentration differential of Na and K inside and outside a cell, is another example of the high discrimination between alkali metal ions that has evolved with biological ligands. The ions are pumped against their concentration gradients by coupling the process to ATP hydrolysis. The mechanism, which is outlined in Fig. 27.7, involves conformational changes induced by ATP-driven protein phosphorylation.
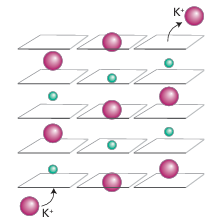
Figure 27.5 Mechanism of transport of K ions through the selectivity filter of the K channel. Green spheres represent water molecules.
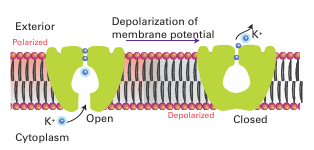
Figure 27.6 Proposed mechanism of action of the K channel. The potential difference across the membrane is sensed by the protein, which causes the pore to open, allowing hydrated ions to enter the cavity. After shedding their hydration sphere, K ions pass up the selectivity filter at rates close to diffusion control.
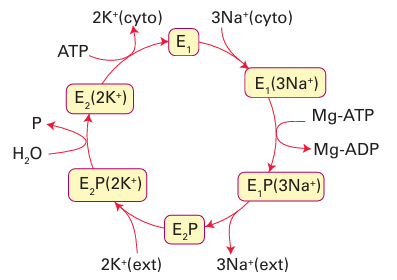
Figure 27.7 General principle of the Na, K-ATPase (the Na pump). Release of two K ions into the cytoplasm is accompanied by binding of ATP (from the cytoplasm) and conversion of the enzyme into state 1, which binds three Na ions from the cytoplasm. A phosphate group (P) is transferred to the enzyme, which opens to the external side, expels three Na ions, then binds two K ions. Release of the phosphate group causes release of K into the cytoplasm and the cycle begins again.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


