
Compartmentalization
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص724-725
الجزء والصفحة:
ص724-725
 2025-10-22
2025-10-22
 69
69
Compartmentalization
Key point: Different elements are strongly segregated inside and outside a cell and among different internal compartments. Compartmentalization is the distribution of elements inside and outside a cell and between different internal compartments. The maintenance of constant ion levels in different bio logical zones is an example of ‘homeostasis’ and it is achieved as a result of membranes being barriers to passive ion flow. An example is the large difference in concentration of K and Na ions across cell membranes. In the cytoplasm, the K concentration may be as high as 0.3 m whereas outside it is usually less than 5 × 10 -3 m. By contrast, Na is abundant outside a cell but scarce inside; indeed, the low intracellular concentration of Na, which has characteristically weak binding to ligands, means that it has few specific roles in biochemistry. Another important example is Ca2, which is almost absent from the cytoplasm (its free concentration is below 1 × 10-7 m) yet is a common cation in the extracellular environment and is concentrated in certain organelles, such as mitochondria. That pH may also vary greatly between different compartments has particularly import ant implications because sustaining a transmembrane proton gradient is a key feature in photosynthesis and respiration.
The distributions of Cu and Fe provide another example: Cu enzymes are often extracellular, that is they are synthesized in the cell and then secreted outside the cell, where they catalyse reactions involving O2. By contrast, Fe enzymes are contained inside the cell. This difference can be rationalized on the basis that the inactive trapped states of these elements are Fe (III) and Cu(I) (or even metallic Cu) and organisms have stumbled on the expediency of keeping Fe in a relatively reducing environment and Cu in a relatively oxidizing environment.
The selective uptake of metal ions has potential industrial applications, for many or-ganisms and organs are known to concentrate particular elements. Thus, liver cells are a good source of cobalamin2 (Co) and milk is rich in Ca. Certain bacteria accumulate Au and thus provide an unusual way for procuring this precious metal. Compartmentalization is an important factor in the design of metal complexes that are used in medicine (Sections 27.17–20). The very small size of bacteria and organelles raises an interesting point about scale, as species present at very low concentrations in very small regions may be represented by only a few individual atoms or molecules. For example, the cytoplasm in a bacterial cell of volume 10 15 dm3 at pH=6 will contain less than 1000 ‘free’ H ions. Indeed, any element nominally present at less than 1 nmol dm3 may be completely absent in individual cases. The word ‘free’ is significant, particularly for metal ions such as Zn2 that are high in the Irving–Williams series; even a eukaryotic cell with a total Zn concentration of 0.1 mmol dm3 may contain very few uncomplexed Zn2 ions.
Two important issues arise in the context of compartmentalization. First, the process requires energy because ions must be pumped against an adverse gradient of chemical potential. However, once a concentration difference has been established, there is a difference in electrical potential across the membrane dividing the two regions. For instance, if the concentrations of K ions on either side of a membrane are [K+]in and [K+]out, then the
1Current geological and geochemical evidence date the advent of atmospheric O2 at between 2.2 and 2.4 Ga ago (1 Ga=109 a). It is likely that this gas arose by the earliest catalytic actions of the photosynthetic Mn cluster described in Section 27.10. 2In nutrition, the common complexes of cobalamin that are ingested are known as vitamin B12.
contribution to the potential difference ∆ across the membrane is
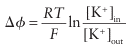
Thisdifference in electrical potential is a way of storing energy, which is released when the ions flood back to their natural concentrations. Second, the selective transport of ions must occur through ion channels built from membrane-spanning proteins, some of which release ions on receipt of an electrical or chemical signal whereas others, the transporters andpumps, transfer ions against the concentration gradient by using energy provided by adenosine triphosphate (ATP) hydrolysis. The selectivity of these channels is exemplified by the highly discriminatory transport of K as distinct from Na (Section 27.3). Proteins, the most important sites for metal ion coordination, are not permanent species but are ceaselessly degraded by enzymes (proteases), releasing both amino acids and metal ions to provide materials for new molecules.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


