

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Clays, pillared clays, and layered double hydroxides
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
636
2025-10-12
91
Clays, pillared clays, and layered double hydroxides
Key point: Sheet-like structures, found in many metal hydroxides and clays, can be constructed from linked metal oxo tetrahedra and octahedra. The diameters of the largest pores found in synthetic zeolites are of the order of 1.2 nm. In an attempt to increase this diameter and allow larger molecules to be absorbed into inorganic structures, chemists have turned to mesoporous materials (Section 25.9) and structures produced by ‘pillaring’ (that is, stacking and connecting together two-dimensional materials). The two-dimensional nature of many d-metal disulfides and some of their intercalation compounds are discussed in Sections 19.9 and 24.10. Similar intercalation reactions when applied to aluminosilicates from the clay family allow the synthesis of large-pore materials. Naturally occurring clays such as kaolinite, hectorite and montmorillonite have layer structures like those shown in Fig. 24.53. The layers are constructed from vertex- and edge-sharing octahedra, MO6, and tetrahedra, TO4, and exist as double layer systems (two layers, one formed from octahedra and one from tetrahedra, Fig. 24.53), as in kaolinite, and triple layer (a central layer based on octahedra sandwiched between two tetrahedrabased layers) systems, as in bentonite. The metal atoms, M and T, contained within the layers, which have an overall negative charge, are typically Si (on tetrahedral sites) and Al (occupying octahedral and tetrahedral sites). Small singly and doubly charged ions, such as Li+ and Mg2+, occupy sites between the layers. These interlayer cations are often hydrated and can readily be replaced by ion exchange. Other materials with similar structures are the layered double hydroxides with structures similar to that of Mg (OH)2, the naturally occurring mineral brucite. In the pillaring of clays, the species exchanged into the interlayer region is selected for size. Ions such as alkylammonium ions and polynuclear hydroxometal ions may replace the alkali metal, as shown schematically in Fig. 24.54. The most widely used pillaring species are of the polynuclear hydroxide type and include Al13O4(OH)283+, Zr4 (OH)n+16-n, and Si8O12(OH)8, the first consists of a central AlO4 tetrahedron surrounded by octahedrally coordinated Al3+ ions as Al (O, OH)6 species. The pillaring process can be followed by powder X-ray diffraction because it leads to expansion of the interlayer spacing, corresponding to an increase in the c lattice parameter. Once an ion such as Al13O4(OH)283+ has been incorporated between the layers, heating the modified clay results in its dehydration and the linking of the ion to the layers (Fig. 24.54). The resulting product is a pillared clay with excellent thermal stability to at least 500ºC. The expanded interlayer region can now absorb large molecules in the same way as zeolites. However, because the distribution of pillaring ions between the layers is difficult to control, the pillared clay structures are less regular than zeolites. Despite this lack of uniformity, pillared clays have been widely studied for their potential as catalysts because they act in a similar way to zeolites, as acid catalysts promoting isomerization and dehydration.
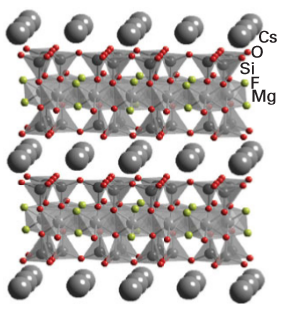
Figure 24.53 Sheet-like structures of the clay hectorite which consist of layers of linked octahedra and tetrahedra centred on, typically, Al, Si, or Mg and separated by cations such as Kor Cs+.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















