

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Chevrel phases and chalcogenide thermoelectrics
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص630-631
2025-10-12
91
Chevrel phases and chalcogenide thermoelectrics
Key point: AChevrel phase has a formula such as Mo6X8 or AXMo6S8, where Se or Temay take the place of S and the intercalated A atom may be a variety of metals such as Li,Mn, Fe, Cd, and Pb. We close this section on sulfide materials with a brief discussion of an interesting class of ternary compounds first reported by R. Chevrel in 1971. These compounds, which illustrate three-dimensional intercalation, have formulas such as Mo6X8 and AxMo6S8; Se or Te may take the place of S and the intercalated A atom may be a variety of metals such as Li, Mn, Fe, Cd, or Pb. The parent compounds Mo6Se8 and Mo6Te8 are prepared by heating the elements at about 1000ºC. A structural unit common to this series is M6S8, which may be viewed as an octahedron of M atoms face-bridged by S atoms, or alternatively as an octahedron of M atoms in a cube of S atoms (Fig. 24.44). This type of cluster is also observed for some halides of the Periods 4 and 5 early d-block elements, such as the [M6X8]4 cluster found in Mo and W dichlorides, bromides, and iodides. Figure 24.45 shows that in the three-dimensional solid the Mo6S8 clusters are tilted relative to each other and relative to the sites occupied by intercalated ions. This tilting allows a secondary donor–acceptor interaction between vacant Mo4dz2 orbitals (which project outward from the faces of the Mo6S8 cube) and a filled donor orbital on the S atoms of adjacent clusters One of the physical properties that has drawn attention to the Chevrel phases is their superconductivity. Superconductivity persists up to 14 K in PbMo6S8, and it also persists to very high magnetic fields, which is of considerable practical interest because many applications involve high fields (over 25 T), for example, for the next generation of NMR instruments. In this respect the Chevrel phases appear to be significantly superior to the newer oxocuprate high-temperature superconductors. Further possible application of Chevrel phases is in the thermoelectric devices used to convert heat into electrical energy or in devices that use electrical energy directly for cooling purposes. The ideal thermoelectric materials have good electrical conductivity and with low thermal conductivities. This requirement often involves designing a material with a combination of structural elements: one that allows for rapid electron transport, a property associated with crystalline solids where there is little electron scattering by the regular placement of atoms, and a second disordered or glassy structural feature, which scatters the vibrational modes responsible for heat transport, thus producing low thermal conductivity. In Chevrel phases the ability to incorporate various cations between the M6X8 blocks that ‘rattle’ around on their sites reduces the material’s thermal conductivity but not at the expense of electronic conductivity.
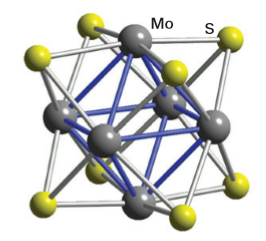
Figure 24.44 The Mo6S8 unit present in a Chevrel phase PbxMo6S8.
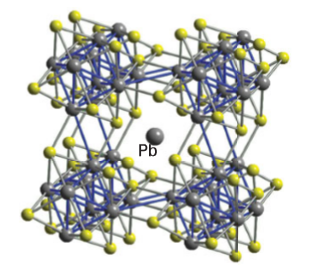
Figure 24.45 The structure of a Chevrel phase showing the canted Mo6S8 units forming a slightly distorted cube around a Pb atom. An Mo atom in one cube can act as the acceptor for an electron pair donated by an S atom in a neighbouring cage. Many other metal chalcogenide phases are being investigated for thermoelectric device applications. The compounds Bi2Te3 and Bi2Se3, in common with many metal chalcogenides, have layer-like structures similar to those of the MS2 phases described in Section 24.10b, and devices built on these materials can be designed to produce good electrical conductivity within the layers but poor thermal conductivity perpendicular to them, leading to useful thermoelectric efficiencies. Another important family of thermoelectric materials are the so-called ‘skutterudites’ (named after the mineral skutterudite, CoAs3) with the general formula Mx (Co, Fe, Ni) (P, As, Sb)3 with various inserted cations, M, such as Ln or Na, as in Na0.25FeSb3. The skutterudite structure is similar to that of ReO3 (Section 24.7) although the CoAs6 octahedra are tilted to produce some large cavities within the structure into which the cations may be inserted. These cations reduce the thermal conductivity of the structure by absorbing heat to rattle around within their cavities, while the high electronic conductivity of the (Co,Fe,Ni)(P,As,Sb)3 network is maintained.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















