

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Carbon clusters
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص356-357
2025-09-04
67
Carbon clusters
Key point: Fullerenes are formed when an electric arc is discharged between carbon electrodes in an inert atmosphere.Metal and nonmetal cluster compounds have been known for decades, but the discovery of the soccer-ball shaped C60 cluster in the 1980s created great excitement in the scientific community and in the popular press. Much of this interest undoubtedly stemmed from the fact that carbon is a common element and there had seemed little likelihood that new molecular carbon structures would be found.When an electric arc is struck between carbon electrodes in an inert atmosphere, a large quantity of soot is formed together with significant quantities of C60 and much smaller quantities of related fullerenes, such as C70, C76, and C84. The fullerenes can be dissolved in a hydrocarbon or halogenated hydrocarbon and separated by chromatography on an alumina column. The structure of C60 has been determined by X-ray crystallography on the solid at low temperature and electron diffraction in the gas phase. The molecule consists of five- and six-membered car bon rings, and the overall symmetry is icosahedral in the gas phase (3).Fullerenes can be reduced to form [60] fulleride salts, C60n (n=1 to 12). Fullerides of alkali metals are solids having com positions such as K3 C60. The structure of K3 C60 consists of a
face-centred cubic array of C60 ions in which K ions occupy the one octahedral and two tetrahedral sites available to each C60 3 ion (Fig. 14.5). The compound is a metallic conductor at room temperature and a superconductor below 18 K. Other Supercon ducting salts include Rb2 CsC60, which has a superconducting transition temperature (Tc) of 33 K, and Cs3 C60, with Tc 40 K. The conductivity of E3C60 compounds can be explained by con sidering that the conduction electrons are donated to the C60 molecules and are mobile because of overlapping C60 molecular orbitals (Section 24.21).Some of the most interesting consequences of fullerene re search have been the identification of carbon nanotubes. Carbon nanotubes consist of one or more concentric cylindrical tubes formed of graphene sheets in which the ends may be closed by fullerene-like caps containing six five-membered rings of atoms. The preparation of nanotubes has stimulated much research and the compounds could ultimately find a wide range of practical applications such as hydrogen storage and catalysis.Graphene has very high electrical conductivity, opacity, and strength and is being investigated for use in electronic devices, bat teries, and for gas storage. It is currently very expensive and it is very difficult to produce perfect layers that give the best electronic performance. The method that produces the cleanest graphene surface is exfoliation, where the surface is mechanically ripped from a graphite crystal. This can be achieved quite simply by using sticky tape, but separating the useful thin flakes from the graph ite debris is time-consuming. A simple and inexpensive route to graphene could revolutionize its use. A step in this direction is a new method that has been developed which can produce gram
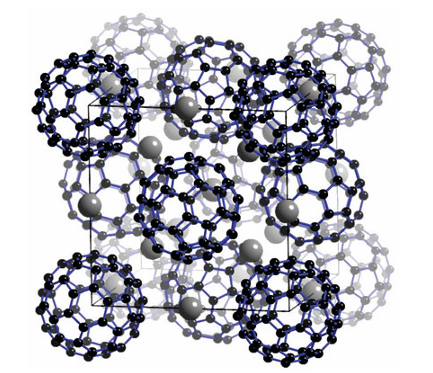
Figure 14.5 The structure of K3C60 . The full cell is face-centred cubic. (The structure of solid C60 itself is shown in Fig. 3.16.)quantities by a simple chemical process: sodium metal is heated with ethanol for three days before being heated rapidly. The result ing solid consists of fused graphene sheets which are then washed and dried to separate them. The quality of the sheets is not as good as those produced by exfoliation, but this may be the first step to large-scale production of perfect graphene flakes.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















