

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Occurrence and extraction
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص296-297
2025-09-04
77
Occurrence and extraction
Key point: The Group 1 elements can be extracted by electrolysis. The name lithium comes from the Greek lithos for stone. The natural abundance of lithium is low, the most abundant minerals being spodumene, LiAlSi2O6, from which lithium is most commonly extracted, and lepidolite, which has the approximate formula K2Li3Al4Si7O21 (F,OH)3. Spodumene is first converted to LiCl and then electrolysed to produce lithium metal. Sodium occurs as the mineral rock salt (NaCl) and in salt lakes and seawater. Sodium chloride makes up 2.6 per cent by mass of the biosphere with the oceans containing 4X1019 kg of the salt. Sodium chloride occurs naturally as the mineral rock salt, the deposits of ancient dried saline lakes. Many of these deposits are underground and are mined in the conventional way. Alternatively, water may be pumped underground to dissolve the rock salt, which is then pumped out as saturated brine solution. The metal is extracted by Down’s process, the electrolysis of molten sodium chloride: 2NaCl(l)→2Na(l)+Cl2(g) The sodium chloride is kept molten at 600°C, a temperature considerably below its melting point of 808°C, by the addition of calcium chloride. A high potential difference, typically between 4 and 8 V, is applied between a carbon anode and an iron cathode immersed in the molten salt. The electrolysis liberates liquid sodium metal at the cathode, which rises to the surface of the cell where it is collected under an inert atmosphere. This process is also used for the industrial production of chlorine, which is generated at the anode. Potassium occurs naturally as potash (K2CO3) and carnallite (KClMgCl2 .6H2O). Natural potassium contains 0.012 per cent of the radioactive isotope 40K, which undergoes decay, with a half-life of 1.25 Ga, to 40Ca and electron capture to 40Ar. The ratio of 40K and 40Ar can be used for dating rocks, specifically the time when the rock solidified, at which point it traps any 40Ar formed. In principle, potassium could be extracted electrolytically but the high reactivity of the element makes this far too hazardous. In stead, molten sodium and molten potassium chloride are heated together and potassium and sodium chloride are formed.
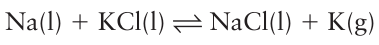
At the temperature of operation, potassium is a vapour and removing it from the system drives the equilibrium to the right. Rubidium (from the Latin rubidus for deep red) and caesium (caesius for sky blue) were discovered by Robert Bunsen in 1861 and named from the colour their salts impart to a flame. Both elements occur as minor constituents of the mineral lepidolite, from which they are obtained as byproducts of the extraction of lithium. Prolonged treatment of lepidolite with sulfuric acid forms the alums of the alkali metals, M2SO4.Al2 (SO4)3. nH2O. The alums are separated by multiple fractional crystallizations and then converted to the hydroxide by reaction with Ba (OH)2 and then to the chloride by ion exchange. The metals are obtained from the molten chloride by reduction with calcium or barium.
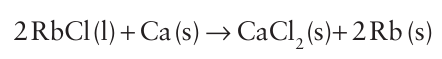
Caesium also occurs as the mineral pollucite, Cs4Al4Si9O26.H2O. The element is extracted from the mineral by leaching with sulfuric acid to form the alum Cs2SO4Al2 (SO4)3.24H2O, which is then converted to the sulfate by roasting with carbon. The chloride is formed by ion exchange and is then reduced with calcium or barium as described above. Caesium metal can also be obtained by the electrolysis of molten CsCN.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















