

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Simple compounds
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص310-311
2025-09-04
74
Simple compounds
Key point: The binary compounds of the Group 2 metals contain the cations of the elements and exhibit predominantly ionic bonding. All the elements occur as M(II) in their simple compounds, which is consistent with their ns2 valence-electron configuration. Apart from Be, their compounds are predominantly ionic. With the exception of Be, the Group 2 elements form ionic (saline) hydrides; the anion present is the hydride ion, H–. In contrast, beryllium hydride adopts a three-dimensional network of linked BeH4 tetrahedra. Magnesium hydride, MgH2, loses hydrogen when heated above 250°C and is being studied as a hydrogen-storage material. The hydrides react with water to produce hydrogen gas. All the elements form halides, MX2, by direct combination of the elements. The halides of the elements other than Be, however, are normally formed from solution, such as by reaction of the metal hydroxide or carbonate with a hydrohalic acid (HX (aq), X = Cl, Br, I) followed by dehydration of the resulting hydrous salt. The fluorides of the larger cations (from Ca to Ba) adopt the (8,4)-coordinate fluorite structure (Fig. 12.2), but MgF2 crystallizes with a rutile structure. The beryllium halides form covalently bonded networks of edge- or corner-linked tetrahedra. Beryllium oxide, BeO, is a white, insoluble solid with the wurtzite structure with (4,4)-coordination, as expected for the small Be2 ion; the oxides of the other Group 2 elements all adopt the rock-salt structure with (6,6)-coordination. Magnesium oxide is insoluble but reacts slowly with water to form Mg (OH)2; likewise, CaO reacts with water to form the partially soluble Ca (OH)2. The oxides of Sr and Ba, SrO and BaO, dissolve in water to form the strongly basic hydroxide solutions:
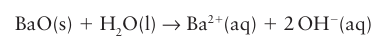
Magnesium hydroxide, Mg(OH)2 , is basic but only very sparingly soluble; beryllium hydroxide, Be (OH)2, is amphoteric and in strongly basic solutions it forms the tetrahy droxyberyllate ion, Be(OH)42- :
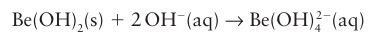
The sulfides can be prepared by direct reaction of the elements and adopt the rock-salt structure for all except Be, which has a sphalerite structure, Fig. 3.34. Beryllium carbide, Be2C, has an antifluorite structure formally containing Be2 and C4 ions. The carbides of the other members of the group have the formula MC2 and contain the dicarbide (acetyl ide) anion, C22–; they react with water to generate ethyne, C2H2. The elements Mg–Ra react directly with nitrogen when heated to produce the nitrides M2N3, which react with water to produce ammonia.With the exception of the fluorides, the salts of singly charged anions are usually soluble in water, although beryllium salts—once again on account of the highly polarizing nature of the Be2 ion—often hydrolyse in aqueous solutions with the formation of [Be(OH2)3 (OH)] and H3O. The radium halides are the least soluble of the group halides: this property is used to extract radium using fractional crystallization. In general, the salts of the Group 2 elements are generally much less soluble in water than those of Group 1 on account of the higher lattice enthalpies of structures containing doubly charged cations, especially when they are in combination with highly charged anions: the carbonates, sulfates, and phosphates are insoluble or only sparingly soluble. The carbonates and sulfates of the Group 2 elements have important roles in natural water systems, rock formation, and as materials for forming hard structures. The carbonates and sulfates are insoluble, as a result of the high lattice energy of structure formed from 2 and 2 ions. The solubility of calcium carbonate increases if CO2 is dissolved in the water, as in rainwater, due to the formation of HCO3 with its lower charge. ‘Temporary hardness’ of water is caused by the presence of magnesium and calcium hydro gencarbonates; the cations are precipitated as carbonates on boiling solutions containing the hydrogen carbonates. Calcium carbonate is widely used by living organisms in the construction of hard structural biomaterials such as shells, bones. When heated, an alkaline earth carbonate decomposes to the oxide, although for Sr and Ba this decomposition process requires temperatures above 800°C. Calcium sulfate is used widely in the construction industry (plaster) and occurs naturally as gypsum, which is the dihydrate, CaSO4.2H2O. The Group 2 cations form complexes with charged polydentate ligands, such as the ana lytically important ethylenediaminetetraacetate ion (edta, see Table 7.1) and crown and crypt ligands. The most important macrocyclic complexes are the chlorophylls, which are porphyrin complexes of Mg and are involved in photosynthesis (Section 27.10d). Beryllium forms an extensive series of organometallic compounds. Alkyl- and aryl magnesium halides are very well known as Grignard reagents and are widely used in synthetic organic chemistry, where they behave as a source of alkyl and aryl anions.
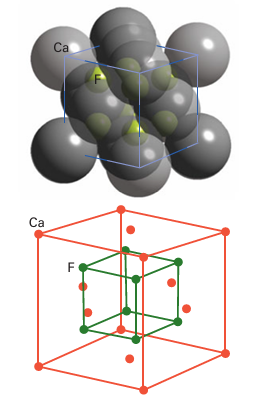
Figure 12.2 The fluorite structure adopted by CaF2, SrF2, BaF2, and SrCl2.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















