

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Diamond and graphite
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص354-356
2025-09-04
73
Diamond and graphite
Key points: Graphite consists of stacked two-dimensional carbon sheets; oxidizing agents or reducing agents may be intercalated between these sheets with concomitant electron transfer. Diamond has the highest known thermal conductivity because its structure (shown in Fig. 14.1) distributes thermal motion in three dimensions very efficiently. The measurement of thermal conductivity is used to identify fake diamonds. Because of its durability, clarity, and high refractive index, diamond is one of the most highly prized gemstones. The ready cleavage of graphite parallel to the planes of atoms (as shown in Fig. 14.2) is largely due to the presence of impurities and accounts for its slipperiness. These graphene planes are widely separated from each other (at 335 pm), indicating that

there are weaker forces between them. These forces are some times, but not very appropriately, called ‘van der Waals forces’ (because in the common impure form of graphite, graphitic ox ide, they are weak, like intermolecular forces), and consequently the region between the planes is called the van der Waals gap. Unlike diamond, graphite is soft and black with a slightly metallic lustre; it is neither durable nor particularly attractive. The conversion of diamond to graphite at room temperature and pressure is spontaneous (∆trs GO =-2.90 kJ mol-1) but does not occur at an observable rate under ordinary conditions: diamonds older than the solar system have been isolated from meteorites. Diamond is the denser phase (3.51 g cm-3 instead of 2.26 g cm-3), so it is favoured by high pressures, and large quantities of diamond abrasive are manufactured commercially by a d-metal-catalysed high-temperature, high-pressure process (Box 14.1). Thin films of boron-doped diamond are piezoresistive (their electrical resistance changes when pressure is applied) and are deposited on silica sur faces for use as high-temperature pressure sensors. The electrical conductivity and many of the chemical proper ties of graphite are closely related to the structure of its delocalized π bonds. Its electrical conductivity perpendicular to the planes is low (5 S cm-1 at 25°C) and increases with increasing temperature, signifying that graphite is a semiconductor in that direction. The electrical conductivity is much higher parallel to the planes (30 kS cm1 at 25°C) but decreases as the temperature is raised, indicating that graphite behaves as a metal, more precisely a semimetal,2 in that direction. This effect is most striking
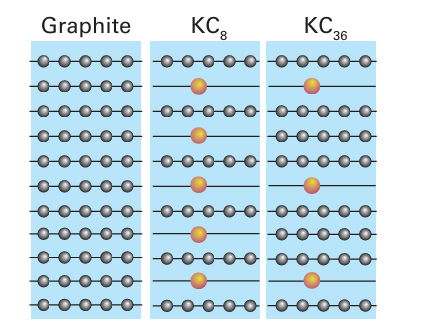
Figure 14.4 Potassium graphite compounds showing two types of alternation of intercalated atoms.
is subsequently removed at high temperatures, a highly flexible form of graphite is formed; this graphite tape is used to make sealing gaskets, valves, and brake linings. The halogens show an alternation effect in their tendency to form intercalation compounds with graphite. Graphite reacts with fluorine to produce ‘graphite fluoride’, a nonstoichiometric species with formula (CF)n (0.59<n<1). This compound is black when n is low in its range and colourless when n ap proaches 1. It is used as a lubricant in high vacuum applications and as the cathode in lithium batteries. At elevated temperatures the products of the reaction also include C2 F and C4 F. Chlorine reacts slowly with graphite to form C8Cl and iodine does not re act at all. By contrast, bromine intercalates readily to give C8 Br, C16 Br, and C20 Br in another example of staging.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















