


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-12-2019
Date: 5-12-2019
Date: 5-12-2019
|
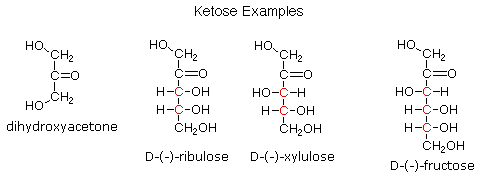
Ketoses
If a monosaccharide has a carbonyl function on one of the inner atoms of the carbon chain it is classified as a ketose. Dihydroxyacetone may not be a sugar, but it is included as the ketose analog of glyceraldehyde. The carbonyl group is commonly found at C-2, as illustrated by the following examples (chiral centers are colored red). As expected, the carbonyl function of a ketose may be reduced by sodium borohydride, usually to a mixture of epimeric products. D-Fructose, the sweetest of the common natural sugars, is for example reduced to a mixture of D-glucitol (sorbitol) and D-mannitol, named after the aldohexoses from which they may also be obtained by analogous reduction. Mannitol is itself a common natural carbohydrate.
Although the ketoses are distinct isomers of the aldose monosaccharides, the chemistry of both classes is linked due to their facile interconversion in the presence of acid or base catalysts. This interconversion, and the corresponding epimerization at sites alpha to the carbonyl functions, occurs by way of an enediol tautomeric intermediate. By clicking on the diagram, an equation illustrating these isomerizations will be displayed.
Because of base-catalyzed isomerizations of this kind, the Tollens' reagent is not useful for distinguishing aldoses from ketoses or for specific oxidation of aldoses to the corresponding aldonic acids. Oxidation by HOBr is preferred for the latter conversion.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|