


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Aqueous solution chemistry and salts of oxoacids of germanium, tin and lead |
|
|
|
Read More
Date: 5-3-2019
Date: 11-6-2020
Date: 10-3-2017
|
Aqueous solution chemistry and salts of oxoacids of germanium, tin and lead
When GeO2 is dissolved in basic aqueous solution, the solution species formed is [Ge(OH)6]2-. With hydrochloric acid, GeO2 forms [GeCl6]2-. Although GeO2 is reduced by H3PO2 in aqueous HCl solution and forms the insoluble Ge(OH)2 when the solution pH is increased, it is possible to retain Ge(II) in aqueous solution under controlled conditions. Thus, 6M aqueous HCl solutions that contain 0.2–0.4 moldm-3 of Ge(II) generated in situ are stable for several weeks.
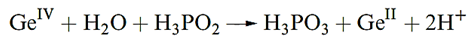
Table 13.1 lists standard reduction potentials for theM4+/ M2+ and M2+/M+ (M=Sn, Pb) couples. The value of Eo(Sn4+ Sn2+) = 0.15V shows that Sn(II) salts in aqueous solution are readily oxidized by O2. In addition, hydrolysis of Sn2+ to species such as [Sn2O(OH)4]2- and [Sn3(OH)4]2+ is extensive. Aqueous solutions of Sn(II) salts are therefore usually acidified and complex ions are then likely to be present, e.g. if SnCl2 is dissolved in dilute hydrochloric acid, [SnCl3] - forms. In alkaline solutions, the dominant species is [Sn(OH)3] -. Extensive hydrolysis of Sn(IV) species in aqueous solution also occurs unless sufficient acid is present to complex the Sn(IV); thus, in aqueous HCl, Sn(IV) is present as [SnCl6]2-. In alkaline solution at high pH, [Sn(OH)6]2- is the main species and salts of this octahedral ion, e.g. K2[Sn(OH)6], can be isolated. In comparison with their Sn(II) analogues, Pb(II) salts are much more stable in aqueous solution with respect to hydrolysis and oxidation. The most important soluble oxosalts are Pb(NO3)2 and Pb(CH3CO2)2. The fact that many water-insoluble Pb(II) salts dissolve in a mixture of [NH4][CH3CO2] and CH3CO2H reveals that Pb(II) is strongly complexed by acetate. Most Pb(II) oxo-salts are, like the halides, sparingly soluble in water; PbSO4 (Ksp = 1.8 × 10-8) dissolves in concentrated H2SO4. For half-reaction as showen in below equation, the fourth-power dependence of the half-cell potential upon [H] immediately explains why the relative stabilities of Pb(II) and Pb(IV) depend upon the pH of the solution.
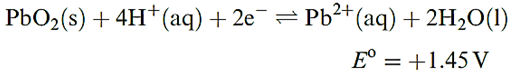
Thus, for example, PbO2 oxidizes concentrated HCl to Cl2, but Cl2 oxidizes Pb(II) in alkaline solution to PbO2. It may be noted that thermodynamically, PbO2 should oxidize water at pH= 0, and the usefulness of the lead–acid battery depends on there being a high overpotential for O2 evolution. Yellow crystals of Pb(SO4)2 may be obtained by electrolysis of fairly concentrated H2SO4 using a Pb anode; however, in cold water, it is hydrolysed to PbO2, as are Pb(IV) acetate and [NH4]2[PbCl6]. The complex ion [Pb(OH)6]2- forms when PbO2 dissolves in concentrated KOH solution, but on dilution of the solution, PbO2 is reprecipitated.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|