


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 31-8-2017
Date: 18-9-2017
Date: 14-9-2017
|
SULFURIC ACID (H2SO4)
Sulfuric acid is the most important and widely used inorganic chemical. The 1994 U.S. production of sulfuric acid was 89.2 billion pounds. (most used industrial chemical). Sulfuric acid is produced by the contact process where sulfur is burned in an air stream to sulfur dioxide, which is catalytically converted to sulfur trioxide. The catalyst of choice is solid vanadium pentoxide (V2O5). The oxidation reaction is exothermic, and the yield is favored at lower temperatures:
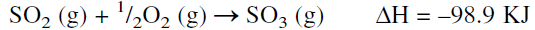
The reaction occurs at about 450°C, increasing the rate at the expense of a higher conversion. To increase the yield of sulfur trioxide, more than one conversion stage (normally three stages) is used with cooling between the stages to offset the exothermic reaction heat. Absorption of SO3 from the gas mixture exiting from the reactor favors the conversion of SO2. The absorbers contain sulfuric acid of 98% concentration which dissolves sulfur trioxide. The unreacted sulfur dioxide and oxygen are recycled to the reactor. The absorption reaction is exothermic, and special coolers are used to cool the acid:
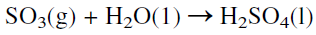



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|