


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-11-2020
Date: 13-6-2019
Date: 13-6-2019
|
Boiling Point Elevation
Since the vapor pressure of the solvent is lowered by the presence of solutes, the temperature at which the vapor pressure equals atmospheric pressure (where the solution boils) is higher than for the pure solvent, as shown in Figure 1.1. Detailed calculations show that the increase in boiling point for a dilute solution is proportional to the total molal concentration of solutes:
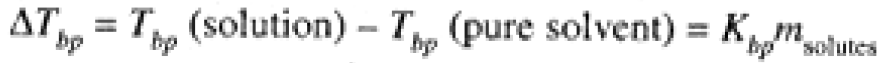 (1.1)
(1.1)
Kbp is the molal boiling-point constant, a property of the solvent and independent of the nature of the solutes; msolutes refers to the total concentration of independent solute particles whether they are neutral molecules or ions. Thus a 0.01 molal NaCl solution has a total solute concentration (Na+ and Cl-) of 0.02 mol/kg. Eq (1-1) is accurate for dilute solutions: For non-ionic solutes, this generally means less than 0.1 mol/kg; for ionic solutes, less than 0.01 mol/kg. For more concentrated solutions, the boiling point is increased but not precisely in proportion to the solute molality.
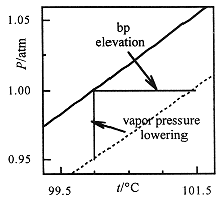
Figure 1.1. Liquid and vapor are at equilibrium along the vapor pressure curves shown for pure water (solid line) and an aqueous solution (dashed line). The vapor pressure is lower for the solution, in accord with Raoult's law, and thus the boiling point is increased (liquids boil at 1 atm).
A boiling point elevation measurement can be used to estimate the molar mass of a solute.
Example 1
3.75 g of a nonvolatile solute was dissolved in 95 g of acetone. The boiling point was 56.50°C compared with 55.95°C for pure acetone. If Kbp = 1.71 K kg/mol for acetone, what is the approximate molar mass of the solute? From Eq. (11-3), we have
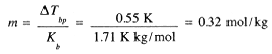
The molar mass thus is
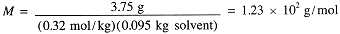



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|