
Basic Crystal Structures
 المؤلف:
Donald A. Neamen
المؤلف:
Donald A. Neamen
 المصدر:
Semiconductor Physics and Devices
المصدر:
Semiconductor Physics and Devices
 الجزء والصفحة:
p 4
الجزء والصفحة:
p 4
 8-5-2017
8-5-2017
 1525
1525
Basic Crystal Structures
Before we discuss the semiconductor crystal, let us consider three crystal structures and determine some of the basic characteristics of these crystals. Figure 1.1 shows the simple cubic, body-centered cubic, and face-centered cubic structures. For these simple structures, we may choose unit cells such that the general vectors 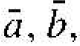 and
and  are perpendicular to each other and the lengths are equal. The simple cubic (sc) structure has an atom located at each corner: the body-centered cubic (bcc) structure has an additional atom at the center of the cube; and the face-centered cubic (fcc) structure has additional atoms on each face plane.
are perpendicular to each other and the lengths are equal. The simple cubic (sc) structure has an atom located at each corner: the body-centered cubic (bcc) structure has an additional atom at the center of the cube; and the face-centered cubic (fcc) structure has additional atoms on each face plane.
By knowing the crystal structure of a material and its lattice dimensions, we can determine several characteristics of the crystal. For example, we can determine the volume density of atoms.

Figure 1.1 Three lattice types: (a) simple cubic. (b) body centered cubic. (c) face-centered cubic.
To find the volume density of atoms in a crystal.
Consider a single-crystal material that is a body-centered cubic with a lattice constant a = 5 A = 5 × 10-8cm . A corner atom is shared by eight unit cells which meet at each corner so that each comer atom effectively contributes one-eighth of its volume to each unit cell. The eight comer atoms then contribute an equivalent of one atom to the unit cell. If we add the body centered atom to the comer atoms, each unit cell contains an equivalent of two atoms.
 الاكثر قراءة في مواضيع عامة في الفيزياء الصلبة
الاكثر قراءة في مواضيع عامة في الفيزياء الصلبة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


