


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-2-2017
Date: 18-6-2019
Date: 20-6-2019
|
Stereoisomerism: geometrical isomers
Distinguishing between cis- and trans-isomers of a square planar complex or between mer- and fac-isomers of an octahedral complex is most unambiguously confirmed by structural determinations using single-crystal X-ray diffraction. Vibrational spectroscopy may also be of assistance. For example, illustrates that the asymmetric stretch for the PtCl2 unit in [Pt(NH3)2Cl2] is IR active for both the trans- and cis-isomers, but the symmetric stretch is IR active only for the cis-isomer.
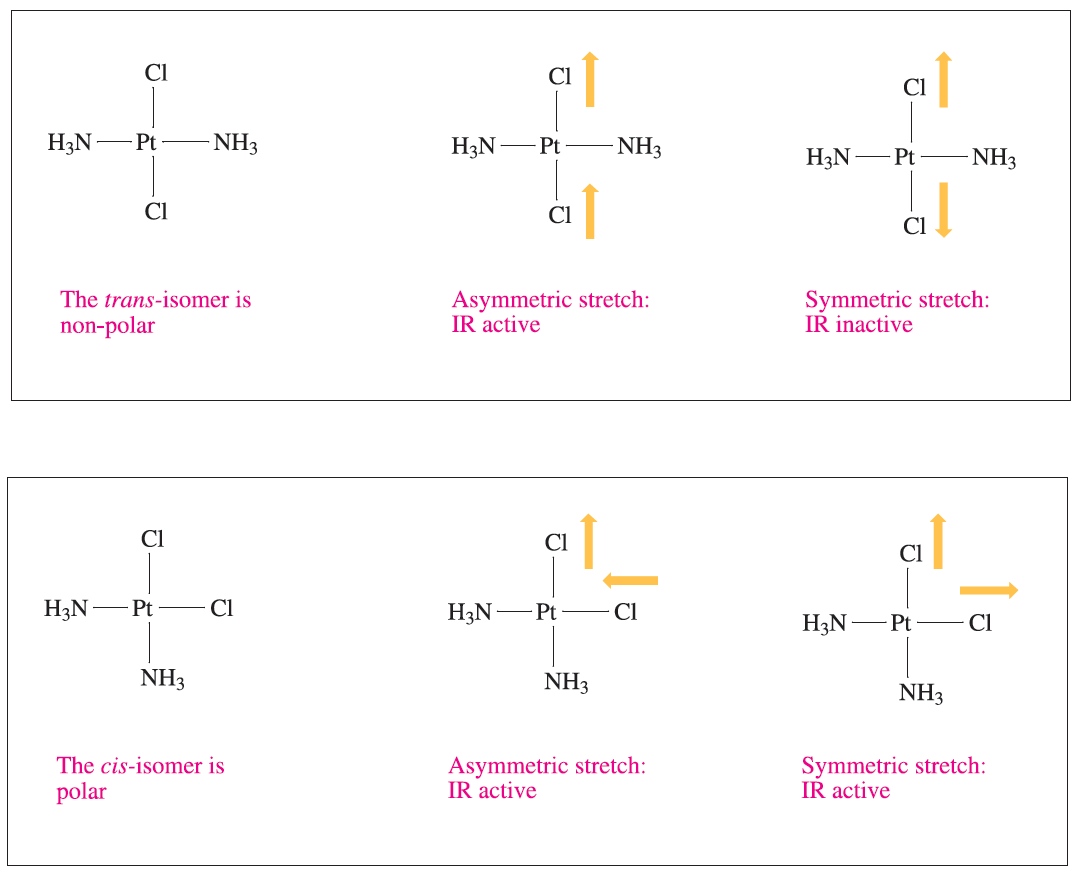
Fig. 1.1 The trans- and cis-isomers of the square planar complex [PtCl2(NH3)2] can be distinguished by IR spectroscopy. The selection rule for an IR active vibration is that it must lead to a change in molecular dipole moment.
The existence of ions or molecules in different structures (e.g. trigonal bipyramidal and square-based pyramidal [Ni(CN)5]3-) is just a special case of geometrical isomerism. In the cases of, for example, tetrahedral and square planar [NiBr2(PBzPh2)2] (Bz = benzyl), the two forms can be distinguished by the fact that they exhibit different magnetic. To complicate matters, square planar [NiBr2(PBzPh2)2] may exist as either trans- or cis-isomers.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|