

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Exothermic reactions: Releasing heat
المؤلف:
John T. Moore, EdD
المصدر:
Chemistry Essentials For Dummies
الجزء والصفحة:
p 91
8-1-2017
2132
Exothermic reactions: Releasing heat
In an exothermic reaction, heat is given off (released) when you go from reactants to products. The reaction between oxygen and methane as you light a gas stove (from the earlier section “Reactants and Products: Reading Chemical Equations”) is a good example of an exothermic reaction.
Even though the reaction gives off heat, you do have to put in a little energy — the activation energy — to get the reaction going. You have to ignite the methane coming out of the burners with a match, lighter, pilot light, or built-in electric igniter.
Imagine that the hypothetical reaction A-B + C → C-A + B is exothermic. The reactants start off at a higher energy state than the products, so energy is released in going from reactants to products. Figure 1.1 shows an energy diagram of this reaction.
In the figure, Ea is the activation energy for the reaction. we show the collision of C and A-B with the breaking of the A-B bond and the forming of the C-A bond at the top of an activation-energy hill. This grouping of reactants at the top of the activationenergy hill is sometimes called the transition state of the reaction.
This transition state shows what bonds are being broken and what bonds are being made. As I show in Figure 1.1, the difference in the energy level of the reactants and the energy level of the products is the amount of energy (heat) that is released in the reaction.
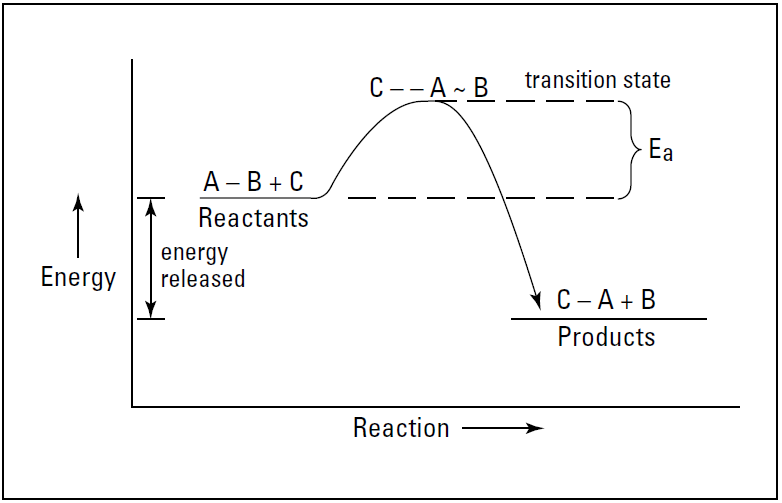
Figure 1.1: Exothermic reaction of A-B + C → C-A + B.
 الاكثر قراءة في الكيمياء الحرارية
الاكثر قراءة في الكيمياء الحرارية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















