


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 16-10-2016
Date: 30-10-2016
Date: 23-10-2016
|
Energy and Reducing Power
ENERGY CARRIERS
Energy enters the biological world through photosynthesis, a process that converts light energy to chemical energy. The sun's light is captured by certain plant pigments that use the energy in chemical reactions. Unfortunately, the energized pigments can enter into only two chemical reactions, although plants have thousands of different reactions in their metabolism. Several theoretical ways exist of transporting energy from energized pigments into endergonic reactions:
1. Allow the pigments to enter into every reaction necessary. A problem is that the
energized pigments are large molecules, so they are not very mobile and never move across membranes (Fig. 1). Furthermore, they are too energetic; they can react with almost anything and would be difficult to control.
2. Allow the energized pigments to make one or several smaller, less energetic intermediates that can be moved and controlled easily. Such a method has evolved: Photo synthetic reactions produce adenosine triphosphate (ATP), an extraordinary molecule. Its high-energy phosphate bonds carry enough energy to force almost any reaction to proceed, and it can enter into almost every reaction for which energy is needed. In those that it does not enter, other energy carriers, often relatives of ATP, are involved; the most frequent is guanosine triphosphate (GTP), which also carries high-energy phosphate bonds.
Although ATP is an essential molecule, it constitutes only a tiny fraction of the plant body. Each molecule is recycled and reused repeatedly, thousands of times per second. ATP is converted to ADP and phosphate by metabolic reactions, but the phosphate can be reattached with a high-energy bond by the reactions of either photosynthesis or respiration. Each molecule is an energy carrier, shuttling between reactions that release energy and those that consume it.

FIGURE 1:The tail of chlorophyll a contains only hydrogen and methyl functional groups, so it is hydrophobic and dissolves into the chloroplast's membrane lipids, immobilizing it. The porphyrin ring system of alternating double and single bonds acts as an antenna that captures light energy. The magnesium atom carries the electrons involved in photosynthesis. If the colored methyl group were an aldehyde group (—CHO), the pigment would be chlorophyll b.
There are three methods by which ADP can be phosphorylated to ATP (Table 1). The first, photophosphorylation, involves light energy in photosynthesis; animals, fog, and non-chlorophyllous plant tissues cannot perform photophosphorylation because they lack the necessary pigments and organelles. Instead, they respire some of the high-energy compounds that they have consumed as food or imported by phloem. Compounds with high-energy phosphate groups are produced, and these compounds force their phosphate onto ADP, making ATP. This is substrate-level phosphorylation. In the last stages of respiration, ADP is phosphorylated to ATP by oxidative phosphorylation. Each process occurs in a distinct site within the cell, and each captures energy from distinct types of exergonic reactions. Substrate-level and oxidative phosphorylation occur in all parts of the plant at all times, but photophosphorylation occurs only in chloroplasts in light.

REDUCING POWER
Earth's atmosphere is about 21% oxygen, so many compounds are found in their oxidized form: carbon as carbon dioxide (CO2), sulfur as sulfate (SO4-2), nitrogen as nitrate (NO3-), and so on. "Oxidized" means that an atom does not carry as many electronsasl and. In carbon dioxide, each oxygen can be considered to have pulled two electrons almost completely away from the carbon, and the carbon is said to be at a +4 oxidation state. This is speaking figuratively; electrons spend more time near the oxygen than they do near the carbon, sulfur, or nitrogen, but they are not torn completely away; these bonds are covalent, not ionic (Table 2).

When electrons are added to an atom, it becomes reduced. Think of a reduction reaction as one that reduces the positive charge on an atom and an oxidation reaction as one that increases the positive charge (Table 3). Oxygen has an electronegativity of 3.5, a strong tendency to pull electrons away from an atom and raise that atom's partial positive charge. But hydrogen becomes more stable by giving up electrons, reducing its partner's partial positive charge. Electrons can be transferred only between atoms or molecules, so each reaction in Table 10.4 is only a "half reaction." Every oxidation occurs simultaneously with a reduction. The full reaction is known as an "oxidation-reduction reaction," or "redox reaction."
Whereas compounds in the environment are predominantly in the oxidized state because of our oxygen-rich atmosphere, most compounds in organisms are in the reduced state. Carbon is often in the form of carbohydrate, where its oxidation state is +0; nitrogen is frequently present as an amino group, NH3 (N-3 H+1 H+1 H+1); and sulfur is present as SH2 (S-2 H+1 H+1). Thus in addition to needing energy, organisms also need reducing power, the ability to force electrons onto compounds. Reducing power is especially important to plants because they take in carbon dioxide and water—the most highly oxidized forms of carbon and hydrogen—and then convert them to carbohydrates, fats, and other compounds that are very reduced. Heterotrophs have less of a problem: When they consume plants, they get compounds that have already been reduced. They do need some reducing power, however, when they synthesize compounds that are very reduced, such as fatty acids.

Just as with energy, an optimum solution for moving and handling reducing power— electrons—is to use small molecules that are semi-stable and mobile. The two molecules used most often are nicotinamide adenine dinucleotide (NAD+; see Figs. 2) and the closely related nicotinamide adenine dinucleotide phosphate (NADP+). Both can pick up a pair of electrons and a proton, thereby becoming reduced to NADH and NADPH. When they reduce a compound by transferring their electrons to it, the proton is released and NAD+ or NADP+ is regenerated. Rather than having a large number of carrier molecules, the cell recycles each molecule, using it thousands of times a second as it shuttles between electron-producing and electron-consuming reactions.

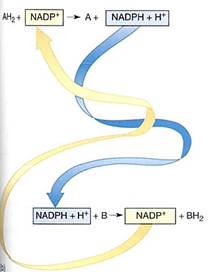
FIGURE 2 :(a) the portion involved in carrying electrons. The oxidized state (NAD+ and NADP+) carries a partial positive charge on the nitrogen and three double bonds in the ring. When it is reduced by two electrons, the positive charge disappears from the nitrogen, numerous bonding orbitals within the ring are changed, and the top carbon picks up a proton. No bonding orbitals are formed between NAD+ and the electron donor, so NADH is free to diffuse away after picking up electrons. Similarly, as it donates electrons to some substrate, the ring bonding orbitals revert to the NAD+ form, none of which binds it to the substrate, so the NAD+ diffuses away. The same is true for NADP+(b) In the upper reaction, two electrons are passed from AH2 to NADP+: AH2 has been oxidized to A, and NADP+ has been reduced to NADPH + H+. The NADPH is free to diffuse to another site, where it passes the two electrons onto another molecule, B, reducing it to BH2 and becoming oxidized back to NADP+.
Because NAD+ and NADP+ take electrons away from other molecules, they are oxidizing agents- they oxidize the material they react with. It is also possible to say that the material has reduced the NAD+ or NADP+. During the process, NADH and NADPH, two strong reducing agents, are formed. They have a powerful tendency to place electrons and other molecules, reducing those molecules and becoming oxidized themselves (Fig. 10.3b) The tendency to accept or donate electrons varies greatly and is known as a molecules redox potential (Table 4). Cells contain a variety of electron carriers that differ in their tendency to accept or donate electrons.

OTHER ELECTRON CARRIERS
Cytochromes. Cytochromes are small proteins that contain a cofactor, heme, which holds an iron atom (Fig. 3); the iron carries electrons and cycles between the +2 and + 3 oxidation states. Cytochromes are intrinsic membrane proteins; they are an integral part of the chloroplast's thylakoid membranes and cannot be removed without destroying the membrane. Consequently, they carry electrons only between sites that are extremely close together within a membrane rather than diffusing throughout the stroma as NADPH does.
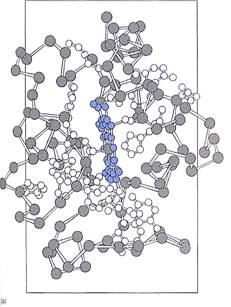

FIGURE 3: (a) In cytochromes, iron is not bonded directly to any amino acid but is held by heme, a boxlike poptynn ring similar to the portion of chlorophyll that holds the magnesium ion. (b) The distirguishirg feature of the quinone class of electron carriers is that each has two ketone groups (he double-bonded oxygen) whose carbon atoms are part of a ring structure.
Plastoquinones. Plastoquinones, like cytochromes, transport electrons over short distances within a membrane (Fig. 10.4b). After they pick up two electrons, they also bind two protons. Their long hydrocarbon tail causes them to be hydrophobic, so they dissolve easily into the lipid component of chloroplast membranes.
Plastocyanin. Like cytochromes, plastocyanin is a small protein that carries electrons on a metal atom, in this case copper. When oxidized, the copper ion is in the +2 oxidation state, but as it picks up the electron, it goes to the +1 oxidation state—it is reduced one level. Plastocyanin is loosely associated with chloroplast membranes; it can move a short distance along the surface, but it does not travel far.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|