


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-7-2019
Date: 22-5-2017
Date: 19-9-2016
|
Axial and Equatorial Bonds in Cyclohexane
The chair conformation of cyclohexane leads to many consequences. for instance, that the chemical behavior of many substituted cyclohexanes is influenced by their conformation. In addition, that simple carbohydrates, such as glucose, adopt a conformation based on the cyclohexane chair and that their chemistry is directly affected as a result.
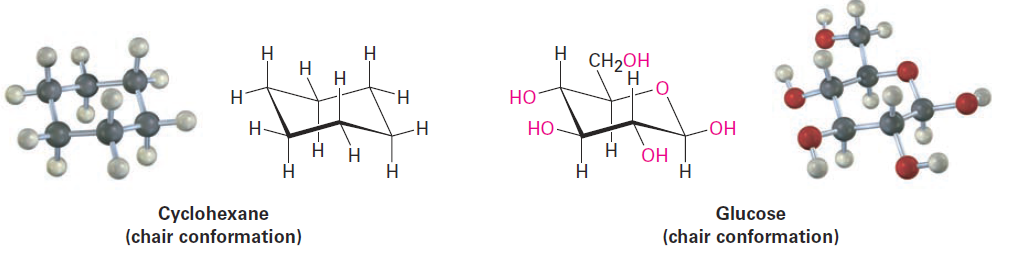
Another trait of the chair conformation is that there are two kinds of positions for substituents on the cyclohexane ring: axial positions and equatorial positions (Figure 1-1). The six axial positions are perpendicular to the ring, parallel to the ring axis, and the six equatorial positions are in the rough plane of the ring, around the ring equator.
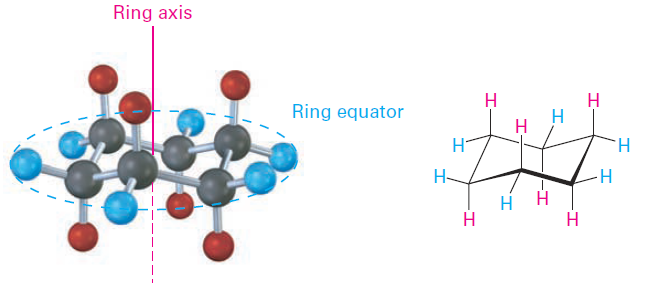
Figure 1-1 Axial and equatorial positions in chair cyclohexane. The six axial hydrogens are parallel to the ring axis, and the six equatorial hydrogens are in a band around the ring equator.
As shown in Figure 1-1, each carbon atom in chair cyclohexane has one axial and one equatorial hydrogen. Furthermore, each face of the ring has three axial and three equatorial hydrogens in an alternating arrangement. For example, if the top face of the ring has axial hydrogens on carbons 1, 3, and 5, then it has equatorial hydrogens on carbons 2, 4, and 6. The reverse is true for the bottom face: carbons 1, 3, and 5 have equatorial hydrogens, but carbons 2, 4, and 6 have axial hydrogens (Figure 1-2).
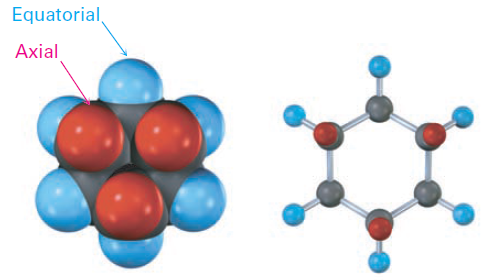
Figure 1-2 Alternating axial and equatorial positions in chair cyclohexane, as shown in a view looking directly down the ring axis. Each carbon atom has one axial and one equatorial position, and each face has alternating axial and equatorial positions.
Note that we haven’t used the words cis and trans in this discussion of cyclohexane conformation. Two hydrogens on the same face of the ring are always cis, regardless of whether they’re axial or equatorial and regardless of whether they’re adjacent. Similarly, two hydrogens on opposite faces of the ring are always trans. Axial and equatorial bonds can be drawn following the procedure in Figure 1-3. Look at a molecular model as you practice.
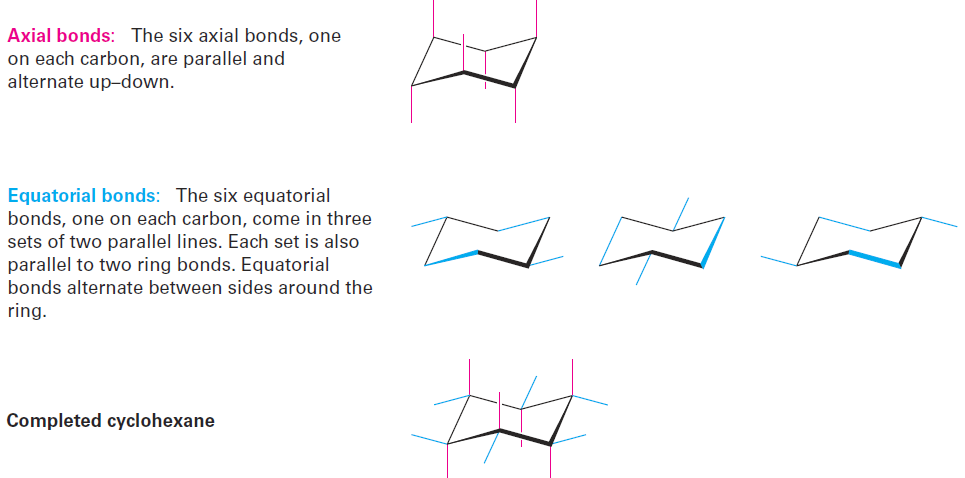
Figure 1-3 A procedure for drawing axial and equatorial bonds in chair cyclohexane.
Because chair cyclohexane has two kinds of positions—axial and equatorial we might expect to find two isomeric forms of a monosubstituted cyclohexane. In fact, we don’t. There is only one methylcyclohexane, one
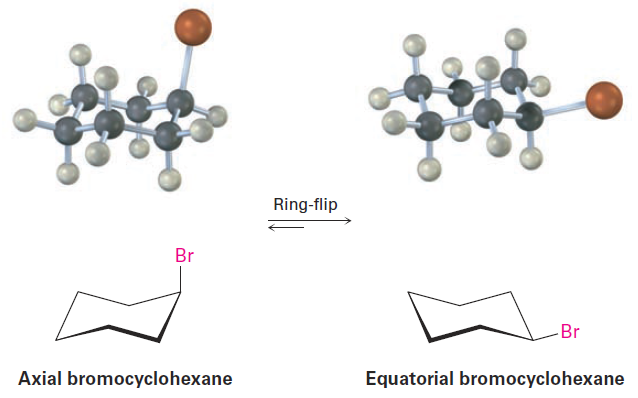
bromocyclohexane, one cyclohexanol (hydroxycyclohexane), and so on, because cyclohexane rings are conformationally mobile at room temperature. Different chair conformations readily interconvert, exchanging axial and equatorial positions. This interconversion, usually called a ring-flip, is shown in Figure 1-4.

Figure 1-4 A ring-flip in chair cyclohexane interconverts axial and equatorial positions. What is axial in the starting structure becomes equatorial in the ringflipped structure, and what is equatorial in the starting structure is axial after ring-flip.
As shown in Figure 1-4, a chair cyclohexane can be ring-flipped by keeping the middle four carbon atoms in place while folding the two end carbons in opposite directions. In so doing, an axial substituent in one chair form becomes an equatorial substituent in the ring-flipped chair form and vice versa. For example, axial bromocyclohexane becomes equatorial bromocyclohexane after a ring-flip. Since the energy barrier to chair–chair interconversion is only about 45 kJ/mol (10.8 kcal/mol), the process is rapid at room temperature and we see what appears to be a single structure rather than distinct axial and equatorial isomers.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|