


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 2024-12-28
Date: 7-3-2016
Date: 14-3-2016
|
Material required for the analyses and Procedure
• Culture media recommended for the tests to be carried out.
• Laboratory incubator(s) or temperature-controlled water baths set to the temperature(s) specified by the test to be performed.
Properly identify all flasks, tubes and plates that will be inoculated, labeling them with the sample code and the standard abbreviation of the culture medium contained in the inoculated container.
1 - Pre-enrichment
In the tests that utilize one single enrichment step, this step is actually not a pre-enrichment procedure, but rather a selective enrichment step. To inoculate the samples, follow the instructions and guidelines provided in the test-specific chapters. The standard dilution of the sample in the broth is 1:10 (one part of sample for nine parts of broth).
2 - Selective enrichment
Carefully agitate the pre-enrichment flask and trans-fer an aliquot to the selective enrichment broth. The proportion between the volume of the aliquot and the volume of the broth is one part of aliquot for ten parts of broth, in the case of most tests. However, there are exceptions, for which the corresponding specific chapter should be consulted. If more than one broth is to be used in the test, transfer an aliquot to the second broth in a similar manner. For incubation of the selective enrichment broth(s), follow the guidelines provided in each specific chapter.
3 - Selective differential plating
From each tube or flask containing enrichment broth, streak a loopful of the culture onto the surface of the differential selective culture medium recommended for the test in the corresponding specific chapter. If more than one plating medium is recommended for the test, repeat this procedure in each medium used. Inoculation should be performed by streak plating, as described below, to obtain isolated colonies in pure culture. Incubation of the plates should be done in the way recommended for the test in the corresponding specific chapters.
3.1 Streak plating technique for obtaining pure cultures
Streaks are made or drawn in the following way, using an inoculation loop: transfer a loopful to a point on the surface of the medium, quite close to the wall of the plate. From this point onwards, move the inocula-tion loop back-and-forth in a kind of zig-zag pattern, drawing or etching lines quite close to each other in half of the plate, as shown in Figure 1. The lines should not touch each other, maintaining a small angle between one line and the next. When half of the plate is reached, flame-sterilize the loop, let cool and touch a point in the inoculated half, close to the wall of the plate. From this point onwards, draw the inoculum out of the inoculated half and form new lines, occupying or covering one quarter of the plate. The new lines should not touch the first and each other. Flame-sterilize the loop again, let cool off and, starting from the second series of streaks, draw again the inoculum to the last, non-inoculated quarter of the plate, etching new lines in the entire remaining area. Do not touch any of the previously made streaks. At each new series of streaks, the inoculum becomes smaller, as it is being gradually depleted in the previous series. This way, it is possible to obtain isolated colonies in the last inoculated quadrant or quarter of the plate.
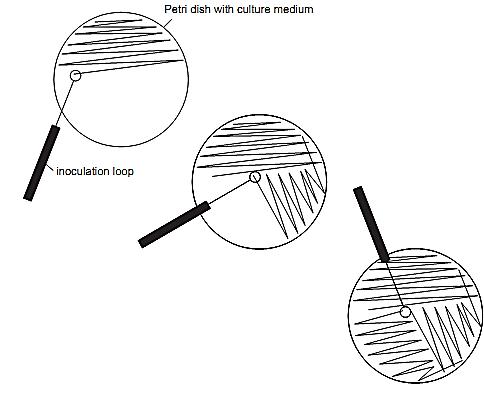
Figure.1 Streak plating technique for obtaining pure cultures.
4 - Selection of colonies and sub-culturing of cultures for confirmation
Confirmation is achieved by subjecting typical colonies of the target microorganism to appropriate assays. In the absence of typical colonies, the test is considered completed and the result reported as absence, even though other types of colonies may be present. When typical colonies are available, the culture should be isolated by transferring a small part of the cell mass to one or more isolation media. The number of colonies that should be isolated for confirmation varies from test to test. In general, at least two typical colonies of each differential plating medium are subjected to confirmation, to increase the probability of isolating one that actually belongs to the target species. For the purpose of isolation, the plate should contain isolated colonies, at least in the quarter that was inoculated last. If this is not the case, a loopful of the culture on the plate should be inoculated again by streak plating onto another plate to obtain isolated colonies. Use the technique described below to subculture the colonies thus obtained on the isolation media, to ensure that pure cultures are transferred.
4.1 Technique for the sub-culturing of pure cultures starting from colonies isolated from plates
The plates normally contain typical colonies, and also atypical colonies of various types, which constitute the competing microflora. To guarantee that the culture taken from a typical colony is indeed pure, the inoculum should be withdrawn with the use of needles (not with inoculation loops). With the tip of the needle, slightly touch the center of the colony at its highest point, and withdraw a minimal quantity of the cell mass. Do not touch any other region of the colony or the culture medium around it, since this would increase the risk of loading contaminants. Never remove the entire colony. A minimum quantity of inoculum, that may even be invisible to the naked eye, is enough to inoculate several isolation media. The media are normally contained in tubes, and may be solid (in slants or not), semi-solid or liquid. The non-inclined semi-solid or solid media are inoculated by stabbing, without reaching or touching the bottom of the tube. The slants are inoculated by streaking the slant and stabbing the butt. The liquid media are inoculated by inserting the needle to a depth close to the bottom, followed by slight agitation. When inoculating several tubes, do not flame-sterilize the needle, nor with-draw a new inoculum between one tube and the next. Incubate the tubes.
5- Confirmation tests
5.1 Gram-staining (Hucker’s method)
Prepare a smear of the culture in the following way: If the culture is in a solid medium, place a drop of a saline solution (NaCl 0.85%) on a clean and dry glass slide and emulsify a loopful of the culture with the saline solution. If the culture is contained in broth, agitate the broth and place a loopful on the glass slide. Wait until the liquid dries naturally and then heat-fix the smear by passing the slide through the flame of a Bunsen burner for three times. Complete the staining procedure the following way: Cover the smear with Hucker’s Crystal Violet Solution and maintain the contact between the smear and the solution for one minute. Wash in running water and cover with Iodine Solution (Lugol) for one minute. Wash in running water and next, flush with ethanol, until no colorant runs off (30 seconds). Wash in running water and cover with Safranin Solution for 30 seconds. Wash in running water and dry. Observe under an optical microscope using an oil immersion objective. Gram-positive bacterial cells will become purple-colored and the Gram-negative cells will turn red. In both cases, both the morphological characteristics of the cells as well as their arrangement and appearance can be observed.
5.2 Spore-staining (Schaeffer-Fulton’s method)
Prepare a smear of the culture, in the same way as described for Gram-staining. Complete the staining procedure the following way: Cover the smear with a 5% aqueous Malachite Green solution and hot stain the smear for five minutes. To heat the malachite green solution, hold the slide over a basin or flask with boiling water. After the recommended contact time, wash with running water and cover with an aqueous 0.5% Safranin Solution for 30 seconds. Wash in running water and dry. Observe under an optical microscope using an oil immersion objective. The spores will turn green and the vegetative cells red.
5.3 Spore-staining (Ashby’s method)
Prepare a smear of the culture, in the same way as described for Gram-staining. Complete the staining procedure the following way: Hold the slide over a basin or flask containing boiling water, until observing drops of condensation water on the lower side of the slide. Cover the smear with a 5% aqueous Malachite Green Solution and stain for one to two minutes. Wash with running water and cover with an aqueous 0.5% Safranin Solution for 20 to 30 seconds. Wash in running water and dry. Observe under an optical micro-scope using an oil immersion objective. The spores will turn green and the vegetative cells red.
5.4 Wet mounts for direct (fresh) microscopic observation
If the culture is contained in a solid medium, place a drop of a saline solution on a clean and dry glass slide and emulsify a loopful of the culture with the saline solution. If the culture is contained in broth, agitate the broth and place a loopful on the glass slide. Cover the liquid with a cover slip and observe immediately under the microscope before the material dries. The best visualization is obtained with a phase contrast micro-scope, but if such an instrument is not available, observation can also be performed using an optical microscope fitted with an oil immersion objective. Direct (or fresh) microscopic observation allows verifying the motility, shape and arrangement of the cells.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|